Exam 6: Some Processes Are Irreversible Thermal Physics
Exam 1: Conservation Laws Constrain Interactions216 Questions
Exam 2: The Laws of Physics Are Universal Newtonian Mechanics147 Questions
Exam 3: The Laws of Physics Are Frame-Independent Relativity124 Questions
Exam 4: Electricity and Magnetism Are Unified333 Questions
Exam 5: Matter Behaves Like Waves Quantum Physics210 Questions
Exam 6: Some Processes Are Irreversible Thermal Physics151 Questions
Select questions type
Which of the following devices are heat engines? In each case, answer T (True) if it is a heat engine, and F (False) if it is not.
-(d) The sun
(True/False)
4.9/5  (45)
(45)
Consider a system consisting of two Einstein solids and in thermal contact. Assume that we know the number of atoms in each solid and . What do we know about the system if we also know the total energy in each of the two objects?
(Multiple Choice)
4.7/5  (36)
(36)
Problems T7T. 3 through T7T. 7 refer to the following cyclic gas process:
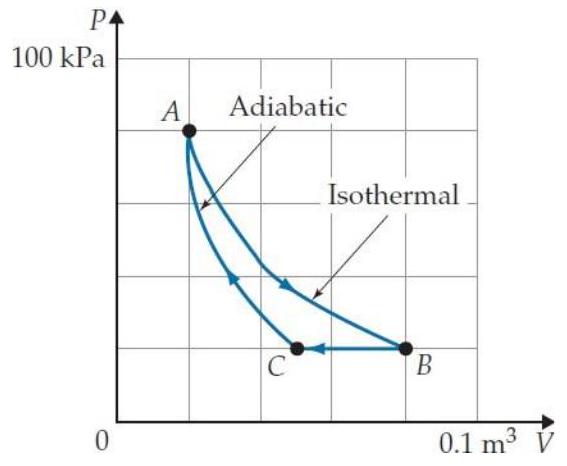 -What are the signs of , and for the process ?
-What are the signs of , and for the process ?
(Multiple Choice)
4.8/5  (33)
(33)
In the situation shown in figure T2.5c, the width of the bell curve at half its peak value is . If we multiply , , and by a factor of 100 , then the peak's width (as you can check) is about . Suppose that we increase each solid's and the system's total energy by another factor of to create solids containing of a mole of atoms (still pretty small objects by everyday standards). Assuming that the trend continues, what will be the approximate width of the combined system's probability bell curve as a fraction of ?
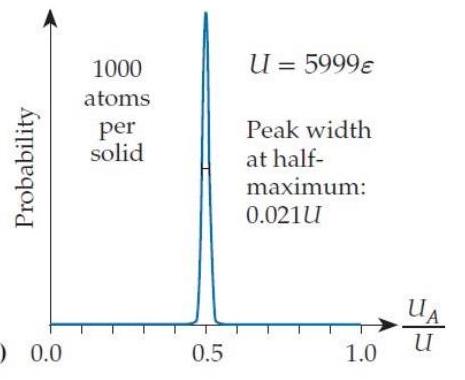
(Multiple Choice)
4.8/5  (41)
(41)
Characterize each of the following processes as being reversible (A) or irreversible (B). (Some answers may be debatable!)
-(b) A ball is dropped and falls freely downward.
(Short Answer)
4.7/5  (35)
(35)
Consider each of the following processes. Is the process consistent (C) or inconsistent (D) with the limitations on equation T8.12 (that be fixed and that the process not involve any energy transfer that is neither heat nor work due to a quasistatic volume change)?
-b) A closed vial of gas is heated with a flame.
(Multiple Choice)
5.0/5  (29)
(29)
Problems T7T. 3 through T7T. 7 refer to the following cyclic gas process:
 -What are the signs of , and for the process ?
-What are the signs of , and for the process ?
(Multiple Choice)
4.8/5  (37)
(37)
Suppose that we allow an ideal gas containing atoms to expand slowly and isothermally to twice its initial volume. By what factor does its multiplicity increase?
(Multiple Choice)
4.8/5  (38)
(38)
molecules of a monatomic gas are mixed with the same number of molecules of a diatomic gas in a container, and the mixture is held at a constant temperature (which is about equal to room temperature). The molecules of gas have mass , and the molecules of gas have mass Which gas has In each case, choose from one of the four answers below.
-(c) the greater total thermal energy , and
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following devices are heat engines? In each case, answer T (True) if it is a heat engine, and F (False) if it is not.
-(b) An automobile engine
(True/False)
4.7/5  (34)
(34)
Suppose that a bubble of helium (a monatomic gas) rising from the bottom of the ocean expands in volume by a factor of 8 by the time it reaches the surface [where the pressure is 1 atmosphere (atm)]. Assume that the bubble rises so fast that it expands essentially adiabatically. What was the pressure on the gas at the depth where it formed (in atmospheres)?
(Multiple Choice)
5.0/5  (46)
(46)
We drop a stone of mass from a height into a bucket of water. What would be a suitable replacement process for this non-quasistatic process?
(Multiple Choice)
4.8/5  (26)
(26)
We slowly heat of water from 5 to . What is its change in entropy? (For water, .)
(Multiple Choice)
4.9/5  (38)
(38)
How does the entropy of of helium gas in a container at room temperature compare to of argon gas in an identical container at the same temperature?
(Multiple Choice)
4.9/5  (43)
(43)
Which of the speeds listed below is the lowest speed where the probability of a gas molecule having that speed is negligible compared to that for ("negligible" meaning less than of its value at ).
(Multiple Choice)
4.7/5  (29)
(29)
Classify the following hypothetical perpetual motion machines as being perpetual motion machines of the first kind or the second kind (B).
-(c) An engine's tank is filled with water. When the engine operates, it slowly freezes the water in the tank, converting the energy released to mechanical energy.
(Multiple Choice)
4.9/5  (41)
(41)
Suppose that you use a super-fast computer to measure a system's macropartition a billion times a second. What is the approximate probability of the least-probable macropartition that you might plausibly see in your lifetime? Select the closest response.
(Multiple Choice)
4.7/5  (36)
(36)
If we increase a one-dimensional container's length by a factor of 100 , by what factor does the value of change at room temperature?
(Multiple Choice)
4.7/5  (44)
(44)
In each of the processes described below, energy flows across the boundary of the object that serves as the subject of each sentence. Is this energy flow heat (A), work (B), or some other kind of energy transfer (E)?
-(d) Your hands get warm when you rub them together.
(Short Answer)
4.9/5  (29)
(29)
Showing 101 - 120 of 151
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)