Exam 6: Some Processes Are Irreversible Thermal Physics
Exam 1: Conservation Laws Constrain Interactions216 Questions
Exam 2: The Laws of Physics Are Universal Newtonian Mechanics147 Questions
Exam 3: The Laws of Physics Are Frame-Independent Relativity124 Questions
Exam 4: Electricity and Magnetism Are Unified333 Questions
Exam 5: Matter Behaves Like Waves Quantum Physics210 Questions
Exam 6: Some Processes Are Irreversible Thermal Physics151 Questions
Select questions type
Using an integral to calculate a sum over (where is an integer) is a good approximation when
(Multiple Choice)
5.0/5  (34)
(34)
Classify the following hypothetical perpetual motion machines as being perpetual motion machines of the first kind or the second kind (B).
-(b) An electric car runs off a battery. When the driver wants to slow down the car, instead of applying the brakes he or she throws a switch that connects the wheels to a generator that converts the car's kinetic energy back to energy in the battery.
(Multiple Choice)
4.8/5  (42)
(42)
In each of the processes described below, energy flows across the boundary of the object that serves as the subject of each sentence. Is this energy flow heat (A), work (B), or some other kind of energy transfer (E)?
-(c) Your car's brakes get hot when used repeatedly.
(Short Answer)
5.0/5  (50)
(50)
In each of the processes described below, energy flows across the boundary of the object that serves as the subject of each sentence. Is this energy flow heat (A), work (B), or some other kind of energy transfer (E)?
-(a) The sun emits light into space.
(Short Answer)
4.9/5  (35)
(35)
The quantity in the expression depends on the temperature of the reservoir.
(True/False)
4.9/5  (38)
(38)
Suppose that we place an aluminum cylinder, a wooden block, and a Styrofoam cup on a table and leave them there for several hours. We then come back into the room and feel each object.
-(a) Which (if any) feels coolest?
(Multiple Choice)
4.8/5  (37)
(37)
Problems T7T. 3 through T7T. 7 refer to the following cyclic gas process:
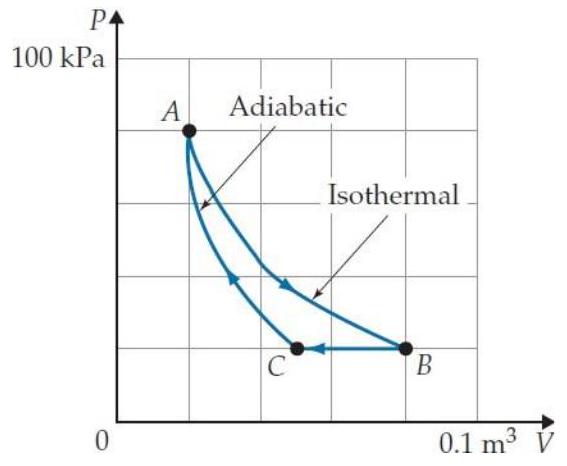 -What are the signs of , and for the process ?
-What are the signs of , and for the process ?
(Multiple Choice)
4.7/5  (41)
(41)
What is the crucial characteristic of an Einstein solid that makes it easier to analyze in the context of this chapter than most other kinds of thermodynamic systems?
(Multiple Choice)
4.9/5  (31)
(31)
molecules of a monatomic gas are mixed with the same number of molecules of a diatomic gas in a container, and the mixture is held at a constant temperature (which is about equal to room temperature). The molecules of gas have mass , and the molecules of gas have mass Which gas has In each case, choose from one of the four answers below.
-(d) the greater pressure ?
(Multiple Choice)
4.7/5  (44)
(44)
In a tub, of water at absorbs from an electric heater. We can reasonably consider the water to be a reservoir in this process.
(True/False)
4.8/5  (42)
(42)
Will a blackbody at temperature emit more photons-perBsecond with energy or with energy ? (Note: The amount of energy emitted per time is not the same as the number of photons emitted per time, though these quantities are related.)
(Multiple Choice)
4.8/5  (36)
(36)
Consider the system where (see figure T2.6). Suppose that we can measure each object's energy to within (the size of one bin). Once the system has reached a macropartition in the most probable bin, it will never spontaneously move to a macropartition outside that bin.
(True/False)
4.8/5  (39)
(39)
If the atmosphere's "optical thickness" depended on the ratio of the concentrations to the first power instead of to the power in equation T10.8a, would this accelerate or brake the warming compared to the model in the text?
Equation T10.8a:}
(Multiple Choice)
4.8/5  (26)
(26)
Physically, why is a gas molecule's speed more likely to be near than near zero?
(Multiple Choice)
4.8/5  (36)
(36)
The heat capacity of any energy storage mode with quantized energy levels goes to zero as the temperature goes to zero.
(True/False)
4.8/5  (34)
(34)
Physically, why is a gas molecule's speed more likely to be near than near ?
(Multiple Choice)
4.9/5  (42)
(42)
Characterize each of the following processes as being reversible (A) or irreversible (B). (Some answers may be debatable!)
-(f) A bowling ball elastically scatters some bowling pins.
(Short Answer)
4.8/5  (27)
(27)
Suppose we hold a sample of helium gas at a constant temperature while we slowly compress it in a cylinder until its volume has decreased by a factor of 4 . During this process, the gas's entropy
(Multiple Choice)
4.8/5  (38)
(38)
molecules of a monatomic gas are mixed with the same number of molecules of a diatomic gas in a container, and the mixture is held at a constant temperature (which is about equal to room temperature). The molecules of gas have mass , and the molecules of gas have mass Which gas has In each case, choose from one of the four answers below.
-(b) the greater average kinetic energy per molecule,
(Multiple Choice)
4.9/5  (33)
(33)
Suppose that a gas expands in a cylinder, pushing back a piston with a speed of (about ). We can reasonably consider such an expansion to be quasistatic.
(True/False)
4.9/5  (37)
(37)
Showing 21 - 40 of 151
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)