Exam 2: Fundamentals of Chemistry
Exam 1: Scope and History of Microbiology55 Questions
Exam 2: Fundamentals of Chemistry63 Questions
Exam 3: Microscopy and Staining56 Questions
Exam 4: Characteristics of Prokaryotic and Eukaryotic Cells63 Questions
Exam 5: Essential Concepts of Metabolism56 Questions
Exam 6: Growth and Culturing of Bacteria58 Questions
Exam 7: Microbial Genetics60 Questions
Exam 8: Gene Transfer and Genetic Engineering57 Questions
Exam 9: An Introduction to Taxonomy: the Bacteria58 Questions
Exam 10: Viruses56 Questions
Exam 11: Eukaryotic Microorganisms and Parasites58 Questions
Exam 12: Sterilization and Disinfection60 Questions
Exam 13: Antimicrobial Therapy56 Questions
Exam 14: Host-Microbe Relationships and Disease Processes55 Questions
Exam 15: Epidemiology and Nosocomial Infections55 Questions
Exam 16: Innate Host Defenses56 Questions
Exam 17: Immunology I: Basic Principles of Adaptive Immunity and Immunization56 Questions
Exam 18: Immunology Ii: Immunological Disorders and Tests60 Questions
Exam 19: Diseases of the Skin and Eyes; Wounds and Bites56 Questions
Exam 20: Urogenital and Sexually Transmitted Diseases57 Questions
Exam 21: Diseases of the Respiratory System58 Questions
Exam 22: Oral and Gastrointestinal Diseases57 Questions
Exam 23: Cardiovascular, lymphatic and Systemic Disease57 Questions
Exam 24: Diseases of the Nervous System56 Questions
Exam 25: Environmental Microbiology55 Questions
Exam 26: Applied Microbiology57 Questions
Select questions type
Ketones,alcohols,aldehydes and organic acids are four of the organic compounds found in all living cells.What do these four classes of organic compounds share?
(Multiple Choice)
4.7/5  (38)
(38)
Charged atomswith electrostatic attraction are generally held together by _____.
(Multiple Choice)
4.9/5  (37)
(37)
When proteins are made up of several polypeptide chains,the arrangement of these chains is referred to as the:
(Multiple Choice)
4.7/5  (25)
(25)
Chemical bonds found in living organisms do not normally include:
(Multiple Choice)
4.8/5  (32)
(32)
The smallest particle of matter that can take part in chemical reactions is:
(Multiple Choice)
4.9/5  (32)
(32)
Solutions made up of molecules that are not chemically bonded and are not limited to specific proportions are called:
(Multiple Choice)
4.9/5  (30)
(30)
Energy is an important chemical currency. How does energy affect electrons in the atom? What is the role of energy in a chemical bond? Name two complex organic molecules that are used by cells for energy.How does the cell release this energy?
(Essay)
4.7/5  (36)
(36)
How many electrons are in the second shell of the chlorine atom? 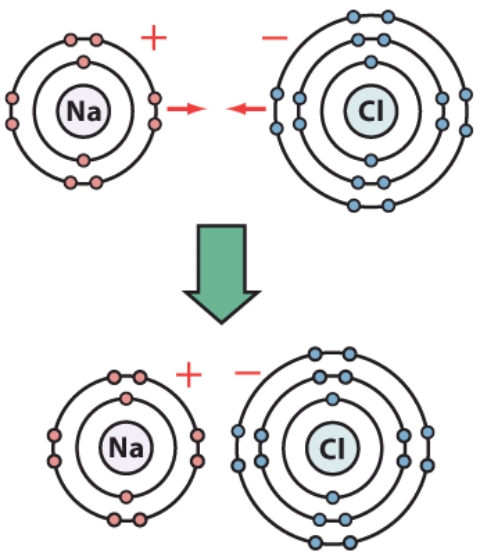
(Multiple Choice)
4.8/5  (33)
(33)
The total number of protons in an atom is equal to its _____.
(Multiple Choice)
4.9/5  (26)
(26)
Showing 41 - 60 of 63
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)