Exam 11: Liquids, Solids, and Intermolecular Forces
Exam 1: Units of Measurement for Physical and Chemical Change179 Questions
Exam 2: Atoms and Elements167 Questions
Exam 3: Molecules, Compounds, and Nomenclature178 Questions
Exam 4: Chemical Reactions and Stoichiometry230 Questions
Exam 5: Gases154 Questions
Exam 6: Thermochemistry156 Questions
Exam 7: The Quantum-Mechanical Model of the Atom173 Questions
Exam 8: Periodic Properties of the Elements127 Questions
Exam 9: Chemical Bonding I: Lewis Theory143 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory168 Questions
Exam 11: Liquids, Solids, and Intermolecular Forces132 Questions
Exam 12: Solutions159 Questions
Exam 13: Chemical Kinetics162 Questions
Exam 14: Chemical Equilibrium123 Questions
Exam 15: Acids and Bases148 Questions
Exam 16: Aqueous Ionic Equilibrium161 Questions
Exam 17: Gibbs Energy and Thermodynamics111 Questions
Exam 18: Electrochemistry126 Questions
Exam 19: Radioactivity and Nuclear Chemistry115 Questions
Exam 20: Organic Chemistry I: Structures109 Questions
Exam 21: Organic Chemistry II: Reactions96 Questions
Exam 22: Biochemistry55 Questions
Exam 23: Chemistry of the Nonmetals50 Questions
Exam 24: Metals and Metallury49 Questions
Exam 25: Transition Metals and Coordination Compounds55 Questions
Select questions type
How much energy must be removed from a 94.4 g sample of benzene (molar mass = 78.11 g mol-1)at 322.0 K to solidify the sample and lower the temperature to 205.0 K? The following physical data may be useful: ΔvapH = 33.9 kJ mol-1
ΔfusH = 9.8 kJ mol-1
Cliq = 1.73 J g-1 °C-1
Cgas = 1.06 J g-1 °C-1
Csol = 1.51 J g-1 °C-1
Tmelting = 279.0 K
Tboiling = 353.0 K
(Multiple Choice)
4.8/5  (44)
(44)
Gold crystallizes in a face-centred cubic structure. What is the edge length of the unit cell if the atomic radius of gold is 144 pm?
(Multiple Choice)
4.8/5  (45)
(45)
Describe the difference between the conduction band and the valence band.
(Essay)
4.9/5  (26)
(26)
Sketch the phase diagram of benzene. Make sure to label the axes and the different phases of benzene. Use the physical data provided below.
melting point = 279 K
boiling point = 353 K
Tc = 562 K
Pc = 49.0 bar
Triple point = 0.05 bar, 279 K
(Essay)
4.9/5  (40)
(40)
Consider the phase diagram shown. Choose the statement below that is TRUE. 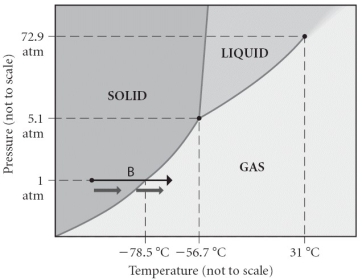
(Multiple Choice)
4.8/5  (30)
(30)
The energy required to increase the surface area of a liquid by a unit amount is called ________.
(Multiple Choice)
4.9/5  (37)
(37)
Place the following substances in order of decreasing vapour pressure at a given temperature. PF5 BrF3 CF4
(Multiple Choice)
4.8/5  (37)
(37)
How much energy is required to heat 87.1 g acetone (molar mass = 58.08 g mol-1)from a solid at -154.0 °C to a liquid at -42. 0°C? The following physical data may be useful: ΔfusH = 7.27 kJ mol-1
Cliq = 2.16 J g-1 °C-1
Cgas = 1.29 J g-1 °C-1
Csol = 1.65 J g-1 °C-1
Tmelting = -95.0 °C
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following compounds exhibits hydrogen bonding?
(Multiple Choice)
4.9/5  (35)
(35)
Place the following compounds in order of increasing strength of intermolecular forces. CO2 F2 NH2CH3
(Multiple Choice)
4.9/5  (34)
(34)
Why does the temperature of a substance stay constant during a phase change such as vaporization?
(Essay)
4.9/5  (39)
(39)
The enthalpy change for converting 10.0 g of ice at -25.0 °C to water at 80.0 °C is ________ kJ. The specific heats of ice, water, and steam are 2.09 J g-1 °C-1, 4.18 J g-1 °C-1, and 1.84 J g-1 °C-1, respectively. For H2O, ΔfusH = 6.01 kJ mol-1, and ΔvapH = 40.67 kJ mol-1.
(Multiple Choice)
4.8/5  (45)
(45)
Showing 101 - 120 of 132
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)