Exam 11: Liquids, Solids, and Intermolecular Forces
Exam 1: Units of Measurement for Physical and Chemical Change179 Questions
Exam 2: Atoms and Elements167 Questions
Exam 3: Molecules, Compounds, and Nomenclature178 Questions
Exam 4: Chemical Reactions and Stoichiometry230 Questions
Exam 5: Gases154 Questions
Exam 6: Thermochemistry156 Questions
Exam 7: The Quantum-Mechanical Model of the Atom173 Questions
Exam 8: Periodic Properties of the Elements127 Questions
Exam 9: Chemical Bonding I: Lewis Theory143 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory168 Questions
Exam 11: Liquids, Solids, and Intermolecular Forces132 Questions
Exam 12: Solutions159 Questions
Exam 13: Chemical Kinetics162 Questions
Exam 14: Chemical Equilibrium123 Questions
Exam 15: Acids and Bases148 Questions
Exam 16: Aqueous Ionic Equilibrium161 Questions
Exam 17: Gibbs Energy and Thermodynamics111 Questions
Exam 18: Electrochemistry126 Questions
Exam 19: Radioactivity and Nuclear Chemistry115 Questions
Exam 20: Organic Chemistry I: Structures109 Questions
Exam 21: Organic Chemistry II: Reactions96 Questions
Exam 22: Biochemistry55 Questions
Exam 23: Chemistry of the Nonmetals50 Questions
Exam 24: Metals and Metallury49 Questions
Exam 25: Transition Metals and Coordination Compounds55 Questions
Select questions type
Why do O, F and N, when bonded to H, form such strong intermolecular attractions to neighbouring molecules? Make sure to be specific.
(Essay)
4.9/5  (43)
(43)
Vanadium crystallizes in a body-centred cubic structure and has an atomic radius of 131 pm. Determine the density of vanadium if the edge length of a bcc structure is 4r/ 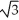 .
.
(Multiple Choice)
4.8/5  (29)
(29)
Identify the compound that does not have dipole-dipole forces as its strongest force.
(Multiple Choice)
4.8/5  (32)
(32)
What is the edge length of a face-centred cubic unit cell made up of atoms having a radius of 128 pm?
(Multiple Choice)
4.7/5  (37)
(37)
Place the following substances in order of decreasing boiling point. H2O N2 CO
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following substances would you predict to have the highest ΔvapH?
(Multiple Choice)
4.7/5  (25)
(25)
Place the following substances in order of decreasing boiling point. N2 O2 H2
(Multiple Choice)
4.9/5  (42)
(42)
Ethyl chloride, C2H5Cl, is used as a local anesthetic. It works by cooling tissue as it vaporizes; its heat of vaporization is 26.4 kJ mol-1. How much heat could be removed by 20.0 g of ethyl chloride?
(Multiple Choice)
4.8/5  (33)
(33)
Determine the vapour pressure (in mbar)of a substance at 29 °C, whose normal boiling point is 76 °C and has a ΔvapH of 38.7 kJ mol-1.
(Multiple Choice)
4.8/5  (43)
(43)
Choose the pair of substances that are most likely to form a homogeneous solution.
(Multiple Choice)
4.8/5  (37)
(37)
Ethanol (C2H5OH)melts at -114 °C. The enthalpy of fusion is 5.02 kJ mol-1. The specific heats of solid and liquid ethanol are 0.97 J g-1 °C-1 and 2.3 J g-1 °C-1, respectively. How much heat (kJ)is needed to convert 25.0 g of solid ethanol at -135 °C to liquid ethanol at -50 °C?
(Multiple Choice)
4.7/5  (41)
(41)
Place the following substances in order of increasing boiling point. CH3CH2OH Ar CH3OCH3
(Multiple Choice)
4.8/5  (41)
(41)
Choose the molecule or compound that exhibits dispersion forces as its strongest intermolecular force.
(Multiple Choice)
4.7/5  (43)
(43)
Showing 61 - 80 of 132
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)