Exam 11: Liquids, Solids, and Intermolecular Forces
Exam 1: Units of Measurement for Physical and Chemical Change179 Questions
Exam 2: Atoms and Elements167 Questions
Exam 3: Molecules, Compounds, and Nomenclature178 Questions
Exam 4: Chemical Reactions and Stoichiometry230 Questions
Exam 5: Gases154 Questions
Exam 6: Thermochemistry156 Questions
Exam 7: The Quantum-Mechanical Model of the Atom173 Questions
Exam 8: Periodic Properties of the Elements127 Questions
Exam 9: Chemical Bonding I: Lewis Theory143 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory168 Questions
Exam 11: Liquids, Solids, and Intermolecular Forces132 Questions
Exam 12: Solutions159 Questions
Exam 13: Chemical Kinetics162 Questions
Exam 14: Chemical Equilibrium123 Questions
Exam 15: Acids and Bases148 Questions
Exam 16: Aqueous Ionic Equilibrium161 Questions
Exam 17: Gibbs Energy and Thermodynamics111 Questions
Exam 18: Electrochemistry126 Questions
Exam 19: Radioactivity and Nuclear Chemistry115 Questions
Exam 20: Organic Chemistry I: Structures109 Questions
Exam 21: Organic Chemistry II: Reactions96 Questions
Exam 22: Biochemistry55 Questions
Exam 23: Chemistry of the Nonmetals50 Questions
Exam 24: Metals and Metallury49 Questions
Exam 25: Transition Metals and Coordination Compounds55 Questions
Select questions type
Place the following substances in order of decreasing boiling point. He Ar H2
(Multiple Choice)
4.8/5  (37)
(37)
Determine the vapour pressure (in mbar)of a substance at 36 °C whose normal boiling point is 84 °C and has a ΔvapH of 22.1 kJ mol-1.
(Multiple Choice)
4.8/5  (45)
(45)
Choose the substance with the highest vapour pressure at a given temperature.
(Multiple Choice)
4.8/5  (29)
(29)
Assign the appropriate labels to the phase diagram shown below. 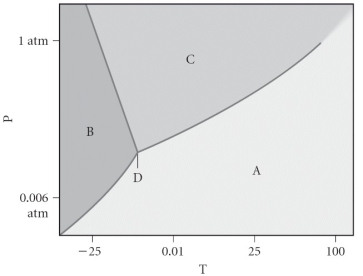
(Multiple Choice)
4.7/5  (36)
(36)
Choose the substance with the lowest surface tension in the liquid phase.
(Multiple Choice)
4.9/5  (34)
(34)
Calculate the total quantity of heat required to convert 25.0 g of liquid CCl4(l)at 35.0 °C to gaseous CCl4 at 76.8 °C (the normal boiling point for CCl4). The specific heat of CCl4(l)is 0.857 J g-1 °C-1, its heat of fusion is 3.27 kJ mol-1, and its heat of vaporization is 29.82 kJ mol-1.
(Multiple Choice)
4.9/5  (37)
(37)
Lithium crystallizes in a body-centred cubic structure. What is the coordination number of each atom?
(Multiple Choice)
4.8/5  (42)
(42)
Based on the figure above, the boiling point of water under an external pressure of 0.316 bar is ________ °C.
(Multiple Choice)
4.9/5  (34)
(34)
NaCl crystallizes in a cubic unit cell with Cl- ions on each corner and each face. How many Na+ and Cl- ions are in each unit cell of NaCl?
(Multiple Choice)
4.9/5  (38)
(38)
Determine the radius of an Al atom (in pm)if the density of aluminum is 2.71 g cm-3. Aluminum crystallizes in a face-centred cubic structure with an edge length of 2 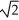 r.
r.
(Multiple Choice)
4.7/5  (36)
(36)
Identify the term used to describe the ability of a liquid to flow against gravity up a narrow tube.
(Multiple Choice)
4.9/5  (39)
(39)
Based on the figure above, the boiling point of diethyl ether under an external pressure of 1.32 bar is ________ °C.
(Multiple Choice)
4.9/5  (38)
(38)
How much energy is required to vaporize 158 g of butane (C4H10)at its boiling point if its ΔvapH is 24.3 kJ mol-1?
(Multiple Choice)
4.9/5  (42)
(42)
How many H- ions are around each Na+ ion in NaH, which has a cubic unit cell with H- ions on each corner and each face?
(Multiple Choice)
4.9/5  (39)
(39)
Choose the substance with the highest viscosity in the liquid phase.
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following compounds exhibits only dispersion and dipole-dipole intermolecular interactions?
(Multiple Choice)
4.8/5  (38)
(38)
Based on the figure above, the boiling point of ethyl alcohol under an external pressure of 0.197 bar is ________ °C.
(Multiple Choice)
4.9/5  (48)
(48)
Showing 41 - 60 of 132
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)