Exam 1: Essentials: Units, Measurements, and Problem Solving
Exam 1: Essentials: Units, Measurements, and Problem Solving101 Questions
Exam 2: Atoms137 Questions
Exam 3: The Quantum Mechanical Model of the Atom141 Questions
Exam 4: Periodic Properties of the Elements144 Questions
Exam 5: Molecules and Compounds202 Questions
Exam 6: Chemical Bonding I138 Questions
Exam 7: Chemical Bonding Ii64 Questions
Exam 8: Chemical Reactions and Chemical Quantities79 Questions
Exam 9: Introduction to Solutions and Aqueous Reactions177 Questions
Exam 10: Thermochemistry145 Questions
Exam 11: Gases185 Questions
Exam 12: Liquids, Solids, and Intermolecular Forces132 Questions
Exam 13: Crystalline Solids and Modern Materials43 Questions
Exam 14: Solutions148 Questions
Exam 15: Chemical Kinetics155 Questions
Exam 16: Chemical Equilibrium141 Questions
Exam 17: Acids and Bases159 Questions
Exam 18: Aqueous Ionic Equilibrium188 Questions
Exam 19: Free Energy and Thermodynamics130 Questions
Exam 20: Electrochemistry148 Questions
Exam 21: Radioactivity and Nuclear Chemistry136 Questions
Exam 22: Organic Chemistry104 Questions
Exam 23: Transition Metals and Coordination Compounds74 Questions
Select questions type
A student weighed 300.0 μg of sulfur in the lab.This is the same mass as
(Multiple Choice)
4.8/5  (42)
(42)
The correct answer (reported to the proper number of significant figures)to the following is
( 1712 - 1615)× ( 8.66 × 7.66)= ________
(Short Answer)
4.9/5  (42)
(42)
The mass of a single arsenic atom is 1.244 × 10-22 g.This is the same mass as
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following are examples of intensive properties?
(Multiple Choice)
4.9/5  (32)
(32)
What does it mean to be an exact number?
Give an example of an exact number.
(Essay)
4.8/5  (37)
(37)
What wavelength of light would you report in units of nm,if the light had a wavelength of 8.80 × 10-10 m?
(Multiple Choice)
5.0/5  (37)
(37)
Identify the unit of measurement which is a SI base unit of measurement.
(Multiple Choice)
5.0/5  (41)
(41)
A mass of mercury occupies 0.750 L.What volume would an equal mass of ethanol occupy? The density of mercury is 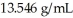 and the density of ethanol is 0.789 g/mL.
and the density of ethanol is 0.789 g/mL.
(Multiple Choice)
4.9/5  (38)
(38)
Determine the mass of a metal cube that has a density of 12.7 g/mL and a volume of 87.63 mL.
(Multiple Choice)
5.0/5  (28)
(28)
How many significant figures are in the measurement,0.0023?
(Multiple Choice)
4.8/5  (44)
(44)
How many µm3 are found in a shape that has a volume of 4.64 × 10-9 cm3?
(Multiple Choice)
4.8/5  (30)
(30)
How many liters of wine can be held in a wine barrel whose capacity is 30.0 gal? 1 gal= 4 qt = 3.7854 L.
(Multiple Choice)
4.8/5  (39)
(39)
A propane molecule contains 3 atoms of carbon.The number 3 represents how many significant figures?
(Multiple Choice)
4.8/5  (38)
(38)
Showing 21 - 40 of 101
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)