Exam 12: Liquids, Solids, and Intermolecular Forces
Exam 1: Essentials: Units, Measurements, and Problem Solving101 Questions
Exam 2: Atoms137 Questions
Exam 3: The Quantum Mechanical Model of the Atom141 Questions
Exam 4: Periodic Properties of the Elements144 Questions
Exam 5: Molecules and Compounds202 Questions
Exam 6: Chemical Bonding I138 Questions
Exam 7: Chemical Bonding Ii64 Questions
Exam 8: Chemical Reactions and Chemical Quantities79 Questions
Exam 9: Introduction to Solutions and Aqueous Reactions177 Questions
Exam 10: Thermochemistry145 Questions
Exam 11: Gases185 Questions
Exam 12: Liquids, Solids, and Intermolecular Forces132 Questions
Exam 13: Crystalline Solids and Modern Materials43 Questions
Exam 14: Solutions148 Questions
Exam 15: Chemical Kinetics155 Questions
Exam 16: Chemical Equilibrium141 Questions
Exam 17: Acids and Bases159 Questions
Exam 18: Aqueous Ionic Equilibrium188 Questions
Exam 19: Free Energy and Thermodynamics130 Questions
Exam 20: Electrochemistry148 Questions
Exam 21: Radioactivity and Nuclear Chemistry136 Questions
Exam 22: Organic Chemistry104 Questions
Exam 23: Transition Metals and Coordination Compounds74 Questions
Select questions type
How much heat is released when 105 g of steam at 100.0°C is cooled to ice at -15.0°C? The enthalpy of vaporization of water is 40.67 kJ/mol,the enthalpy of fusion for water is 6.01 kJ/mol,the molar heat capacity of liquid water is 75.4 J/(mol ∙ °C),and the molar heat capacity of ice is 36.4 J/(mol ∙ °C).
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
C
Consider the phase diagram below.If the dashed line at 1 atm of pressure is followed from 100 to 500°C,what phase changes will occur (in order of increasing temperature)? 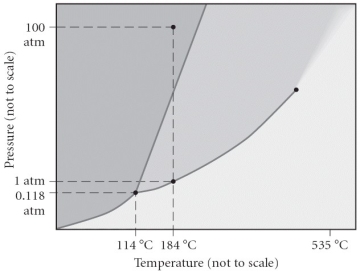
Free
(Multiple Choice)
4.7/5  (36)
(36)
Correct Answer:
D
The normal boiling point for H2Se is higher than the normal boiling point for H2S .This can be explained by
Free
(Multiple Choice)
4.7/5  (38)
(38)
Correct Answer:
B
Which of the following must be overcome during the boiling of acetonitrile,CH3CN?
1)dispersion forces
2)dipole-dipole forces
3)hydrogen bonds
(Multiple Choice)
4.9/5  (37)
(37)
How much energy must be removed from a 94.4 g sample of benzene (molar mass= 78.11 g/mol)at 322.0 K to solidify the sample and lower the temperature to 205.0 K? The following physical data may be useful.
ΔHvap = 33.9 kJ/mol
ΔHfus = 9.8 kJ/mol
Cliq = 1.73 J/g°C
Cgas = 1.06 J/g°C
Csol = 1.51 J/g°C
Tmelting = 279.0 K
Tboiling = 353.0 K
(Multiple Choice)
4.9/5  (31)
(31)
Sketch the phase diagram of benzene.Make sure to label the axes and the different phases of benzene.Use the physical data provided below.
melting point = 279 K
boiling point = 353 K
Tc = 562 K
Pc = 48.4 atm
Triple Point = 0.05 atm,279 K
(Essay)
4.8/5  (42)
(42)
The enthalpy change for converting 10.0 g of ice at -25.0°C to water at 80.0°C is ________ kJ.The specific heats of ice,water,and steam are 2.09 J/gK,4.18 J/gK,and 1.84 J/gK,respectively.For H2O,ΔHfus = 6.01 kJ/mol,and ΔHvap = 40.67 kJ/mol.
(Multiple Choice)
4.8/5  (34)
(34)
Determine the vapor pressure (in torr)of a substance at 36°C,whose normal boiling point is 84°C and has a ΔHvap of 22.1 kJ/mol.
(Multiple Choice)
4.8/5  (34)
(34)
The set of conditions at which point a phase boundary no longer exists is know as the
(Multiple Choice)
4.8/5  (42)
(42)
Why does the temperature of a substance stay constant during a phase change such as vaporization?
(Essay)
4.8/5  (47)
(47)
Describe the differences in the meniscuses of water and mercury.
(Multiple Choice)
4.8/5  (33)
(33)
The normal boiling point of diethyl ether is 34.6°C.At a pressure of 1.3 atm,the boiling point
(Multiple Choice)
4.8/5  (30)
(30)
Place the following compounds in order of increasing strength of intermolecular forces. CO2 H2 H2O
(Multiple Choice)
4.8/5  (39)
(39)
Showing 1 - 20 of 132
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)