Exam 3: The Quantum Mechanical Model of the Atom
Exam 1: Essentials: Units, Measurements, and Problem Solving101 Questions
Exam 2: Atoms137 Questions
Exam 3: The Quantum Mechanical Model of the Atom141 Questions
Exam 4: Periodic Properties of the Elements144 Questions
Exam 5: Molecules and Compounds202 Questions
Exam 6: Chemical Bonding I138 Questions
Exam 7: Chemical Bonding Ii64 Questions
Exam 8: Chemical Reactions and Chemical Quantities79 Questions
Exam 9: Introduction to Solutions and Aqueous Reactions177 Questions
Exam 10: Thermochemistry145 Questions
Exam 11: Gases185 Questions
Exam 12: Liquids, Solids, and Intermolecular Forces132 Questions
Exam 13: Crystalline Solids and Modern Materials43 Questions
Exam 14: Solutions148 Questions
Exam 15: Chemical Kinetics155 Questions
Exam 16: Chemical Equilibrium141 Questions
Exam 17: Acids and Bases159 Questions
Exam 18: Aqueous Ionic Equilibrium188 Questions
Exam 19: Free Energy and Thermodynamics130 Questions
Exam 20: Electrochemistry148 Questions
Exam 21: Radioactivity and Nuclear Chemistry136 Questions
Exam 22: Organic Chemistry104 Questions
Exam 23: Transition Metals and Coordination Compounds74 Questions
Select questions type
If two electrons in the same atom have the same value of "l",they are
Free
(Multiple Choice)
5.0/5  (41)
(41)
Correct Answer:
A
Calculate the wavelength (in nm)of the blue light emitted by a mercury lamp with a frequency of 6.88 × 1014 Hz.
Free
(Multiple Choice)
4.9/5  (44)
(44)
Correct Answer:
B
Give all possible values of l for a n=5 sublevel.
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
C
Calculate the energy of the red light emitted by a neon atom with a wavelength of 703.2 nm.
(Multiple Choice)
4.8/5  (46)
(46)
When a wave encounters an obstacle or a slit that is comparable in size to its wavelength,it bends around it.This characteristic is called
(Multiple Choice)
4.9/5  (47)
(47)
How many photons are contained in a burst of yellow light (589 nm)from a sodium lamp that contains 616 kJ of energy?
(Multiple Choice)
4.9/5  (39)
(39)
Calculate the energy of the orange light emitted,per photon,by a neon sign with a frequency of 4.89 × 1014 Hz.
(Multiple Choice)
4.8/5  (24)
(24)
Determine the velocity of a marble (m = 7.75 g)with a wavelength of 3.46 × 10-33 m.
(Multiple Choice)
4.8/5  (34)
(34)
For a hydrogen atom,which electronic transition would result in the emission of a photon with the highest energy?
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following visible colors of light has the highest frequency?
(Multiple Choice)
4.8/5  (42)
(42)
Determine the end (final)value of n in a hydrogen atom transition,if the electron starts in 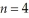 and the atom emits a photon of light with a wavelength of 486 nm.
and the atom emits a photon of light with a wavelength of 486 nm.
(Multiple Choice)
4.9/5  (36)
(36)
Place the following types of electromagnetic radiation in order of increasing wavelength. ultraviolet light gamma rays microwaves
(Multiple Choice)
4.9/5  (28)
(28)
Which of the following transitions represent the emission of a photon with the largest energy?
(Multiple Choice)
4.9/5  (42)
(42)
Calculate the wavelength (in nm)of a the red light emitted by a neon sign with a frequency of 5.00 × 1014 Hz.
(Multiple Choice)
4.9/5  (40)
(40)
Choose the transition (in a hydrogen atom)below that represents the absorption of the shortest wavelength photon.
(Multiple Choice)
4.7/5  (40)
(40)
Determine the mass of a ball with a velocity of 40.0 m/s and a wavelength of 8.92 × 10-34 m.
(Multiple Choice)
4.9/5  (31)
(31)
Showing 1 - 20 of 141
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)