Exam 10: Quantity Relationships in Chemical Reactions
Exam 2: Matter and Energy50 Questions
Exam 3: Measurement and Chemical Calculations48 Questions
Exam 4: Introduction to Gases56 Questions
Exam 5: Atomic Theory: The Nuclear Model of the Atom48 Questions
Exam 6: Chemical Nomenclature45 Questions
Exam 7: Chemical Formula Relationships45 Questions
Exam 8: Chemical Reactions44 Questions
Exam 9: Chemical Change52 Questions
Exam 10: Quantity Relationships in Chemical Reactions42 Questions
Exam 11: Atomic Theory: The Quantum Model of the Atom50 Questions
Exam 12: Chemical Bonding45 Questions
Exam 13: Structure and Shape47 Questions
Exam 14: The Ideal Gas Law and Its Applications47 Questions
Exam 15: Gases, Liquids, and Solids45 Questions
Exam 16: Solutions47 Questions
Exam 17: Acidbase Proton Transferreactions45 Questions
Exam 18: Chemical Equilibrium45 Questions
Exam 19: Oxidationreduction Electron Transferreactions45 Questions
Exam 20: Nuclear Chemistry50 Questions
Exam 21: Organic Chemistry45 Questions
Exam 22: Biochemistry45 Questions
Select questions type
A typical diet provides about 2000 food Calories per day,or 2000 kilocalories.Calculate the equivalent number of joules.
(Multiple Choice)
4.9/5  (35)
(35)
Barium chloride was used to precipitate silver chloride from a solution of silver nitrate.What mass of barium chloride had to react if 0.635 g of silver chloride formed?
(Multiple Choice)
4.8/5  (40)
(40)
How many moles of water are produced by burning 5.77 moles of C4H10?
(Multiple Choice)
4.7/5  (28)
(28)
In a general chemistry laboratory experiment,a student produces 2.73 g of a compound.She calculates the theoretical yield as 3.40 g.What is the percent yield?
(Multiple Choice)
4.8/5  (31)
(31)
In the reaction 2 AgI + HgI2 → Ag2HgI4,2.00 g of AgI and 3.50 g of HgI2 were used.What is the limiting reactant?
(Multiple Choice)
4.8/5  (35)
(35)
In reacting aluminum carbonate with hydrochloric acid according to the equation
Al2(CO3)3 + 6 HCl → 2 AlCl3 + 3 H2O + 3 CO2,57.6 mg of carbon dioxide was formed.What mass of aluminum carbonate reacted to form this amount of carbon dioxide?
(Multiple Choice)
4.7/5  (31)
(31)
If the reaction N2 + 3 H2 → 2 NH3 is carried out using 1.40 g of N2 and 0.400 g of H2,what mass of excess reactant will remain?
(Multiple Choice)
4.9/5  (27)
(27)
Consider the following thermochemical equation: CaO(s)+ H2O( 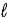 )→ Ca(OH)2(s)ΔH = -66.5 kJ Which of the following statements is/are correct?
i.The reaction is exothermic
ii.The reaction releases 66.5 kJ per gram of Ca(OH)2(s)formed
iii.Alternatively,the ΔH term could be added to the product side of the equation
)→ Ca(OH)2(s)ΔH = -66.5 kJ Which of the following statements is/are correct?
i.The reaction is exothermic
ii.The reaction releases 66.5 kJ per gram of Ca(OH)2(s)formed
iii.Alternatively,the ΔH term could be added to the product side of the equation
(Multiple Choice)
4.9/5  (42)
(42)
The thermochemical equation describing the heat change in the decomposition of limestone,CaCO3,is given by CaCO3(s)+ 176 kJ → CaO(s)+ CO2(g).What is the heat change that accompanies the decomposition of 25.0 g of limestone?
(Multiple Choice)
4.9/5  (36)
(36)
Consider the hypothetical reaction A + 2 B → AB2.If 2 moles of A and 5 moles of B are placed in a container an allowed to react,how many moles of AB2 will be formed and how many moles of which reactant will remain unreacted?
(Multiple Choice)
4.7/5  (41)
(41)
In the reaction P4 + 5 O2 → 2 P2O5,in order the convert from moles of any substance to moles of another substance as shown by the block diagram.  What is the conversion factor needed?
What is the conversion factor needed?
(Multiple Choice)
4.8/5  (47)
(47)
A chemical reaction resulted in 1.376 g of product,which represented a 78.5 percent yield for the reaction.What is the theoretical yield?
(Multiple Choice)
4.9/5  (30)
(30)
In the combustion of natural gas according to the equation CH4 + 2 O2 → CO2 + 2 H2O,how many grams of water are formed during the combustion of 0.264 mole of CH4?
(Multiple Choice)
4.9/5  (38)
(38)
Formation of two moles of hydrogen chloride gas from the gases hydrogen and chlorine is a reaction for which ΔH is -185 kJ.Which of the following statements is correct?
i.The reaction is exothermic.
ii.Enthalpy of the system decreases.
iii.Heat is a "product".
(Multiple Choice)
4.8/5  (43)
(43)
The reaction 2 Sb + 3 Cl2 → 2 SbCl3 has an 88.3% yield.If 4.07 g of SbCl3 is desired,what mass of chlorine should be used?
(Multiple Choice)
4.8/5  (37)
(37)
Ammonium sulfate can be made by the reaction H2SO4 + 2 NH3 → (NH4)2SO4.What quantity of ammonium sulfate will result from the reaction of 5.00 kg of ammonia and 20.0 kg of sulfuric acid?
(Multiple Choice)
4.9/5  (47)
(47)
What is the theoretical yield of phosphorus triiodide when 4.50 g of phosphorus is combined with 45.0 g of iodine? The compound is made by the direct combination of the elements.
(Multiple Choice)
4.7/5  (37)
(37)
In the reaction CaCN2 + 3 H2O → CaCO3 + 2 NH3,how many kilograms of NH3 will be produced when 25.0 kg of CaCN2 react with excess water?
(Multiple Choice)
4.8/5  (37)
(37)
Silver nitrate and magnesium chloride solutions are mixed.What is the Per expression that allows one to convert moles of silver nitrate to moles of magnesium chloride?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 21 - 40 of 42
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)