Exam 8: Quantities in Chemical Reactions
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases123 Questions
Exam 12: Liquids, solids, and Intermolecular Forces115 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry109 Questions
Exam 19: Biochemistry110 Questions
Select questions type
If it takes 2 cups of milk and 1 cup of cocoa mix to make three servings of hot chocolate,and you only have 1 cup of each,then you cannot make any hot chocolate.
(True/False)
4.8/5  (36)
(36)
Which ingredient is the limiting reactant if you have 5 cups of flour,9 eggs and 3 tbs of oil? Given: 2 cups flour + 3 eggs + 1 tbs oil → 4 waffles
(Multiple Choice)
4.9/5  (37)
(37)
How many grams of calcium phosphate are theoretically produced if we start with 3.40 moles of Ca(NO3)2 and 2.40 moles of Li3PO4?
Reaction: 3Ca(NO3)2 + 2Li3PO4 → 6LiNO3 + Ca3(PO4)2
(Multiple Choice)
4.8/5  (42)
(42)
How many grams of water are needed to react with 27.2 grams of Li2O?
Given: Li2O + H2O → 2 LiOH
(Multiple Choice)
4.8/5  (35)
(35)
How many moles of sodium metal are needed to make 3.6 moles of sodium chloride? Given the reaction:
2Na + Cl2 → 2NaCl
(Multiple Choice)
4.8/5  (33)
(33)
If the theoretical yield of a reaction is 144 grams and the actual yield of the reaction is 72 grams,the percent yield of the reaction is 200%.
(True/False)
4.8/5  (40)
(40)
Iron metal reacts with oxygen to produce iron(III)oxide.If you have 12.0 moles of iron for complete reaction,you need:
(Multiple Choice)
4.7/5  (32)
(32)
Many metals react with halogens to give metal halides.For example, 2 Al (s)+ 3 Cl2(g)→ 2 AlCl3 (s)
If you begin with 13.5 g of aluminum:
(Multiple Choice)
4.7/5  (36)
(36)
Consider the following generic chemical equation: 2W + 3X → 3Y + Z When 5 units of W and 6 units of X are allowed to react,the limiting reactant would be:
(Multiple Choice)
4.8/5  (36)
(36)
Greenhouse gases affect the temperature of the earth by blocking sunlight from reaching earth.
(True/False)
4.8/5  (33)
(33)
One of the advantages of burning fossil fuels is that it produces O2 for humans to breathe.
(True/False)
4.9/5  (38)
(38)
Diatomic N2 can react with diatomic H2 to form ammonia (NH3).The balanced chemical equation is:
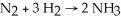 If 6 moles of H2 totally reacted with more than enough N2,how many moles of ammonia would be expected to form?
If 6 moles of H2 totally reacted with more than enough N2,how many moles of ammonia would be expected to form?
(Multiple Choice)
4.8/5  (38)
(38)
The average global temperature depends on all factors EXCEPT what?
(Multiple Choice)
4.8/5  (35)
(35)
If the theoretical yield of a reaction is 42.0 grams of product and the percent yield is 75%.How many grams were actually produced?
(Multiple Choice)
4.7/5  (41)
(41)
Given the recipe: 2 cups flour + 1 egg + 3 oz blueberries → 4 muffins
If you have 5 cups of flour,3 eggs and plenty of blueberries,the limiting reactant is the eggs.
(True/False)
4.8/5  (34)
(34)
Given that 4 NH3 + 5 O2 → 4 NO + 6 H2O,if 3.00 mol NH3 were made to react with excess of oxygen gas,the amount of H2O formed would be:
(Multiple Choice)
4.7/5  (41)
(41)
How many moles of chlorine gas are needed to make 0.6 moles of sodium chloride? Given the reaction:
2Na + Cl2 → 2NaCl
(Multiple Choice)
4.8/5  (43)
(43)
Thermal energy flows into the reaction and out of the surroundings in an endothermic reaction.
(True/False)
4.9/5  (42)
(42)
Showing 81 - 100 of 115
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)