Exam 8: Quantities in Chemical Reactions
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases123 Questions
Exam 12: Liquids, solids, and Intermolecular Forces115 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry109 Questions
Exam 19: Biochemistry110 Questions
Select questions type
The theoretical yield is the amount of each reactant needed in order to make the maximum amount of product.
(True/False)
4.7/5  (32)
(32)
Given the chemical equation: 2 Ca + O2 → 2 CaO,
if 2 moles of CaO are formed in this reaction,then 2 moles of O2 must have reacted.
(True/False)
4.8/5  (31)
(31)
A balanced chemical equation used to prepare ammonium carbonate,(NH4)2CO3 ,is: 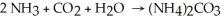 Which choice of reactant quantities shown below would result in the greatest amount of ammonium carbonate being formed?
Which choice of reactant quantities shown below would result in the greatest amount of ammonium carbonate being formed?
(Multiple Choice)
5.0/5  (41)
(41)
How many grams of the excess reactant are left over according to the reaction below given that you start with 10.0 g of Al and 19.0 grams of O2?
Reaction: 4Al + 3O2 → 2Al2O3
(Multiple Choice)
4.8/5  (40)
(40)
In order to determine the limiting reactant in a particular reaction,one must know each of the following EXCEPT:
(Multiple Choice)
4.8/5  (33)
(33)
Diatomic O2 can react with the element magnesium to form magnesium oxide (MgO).The balanced chemical equation is:
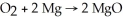 If 4 moles of magnesium totally reacted with more than enough O2,how many moles of MgO would be expected to form?
If 4 moles of magnesium totally reacted with more than enough O2,how many moles of MgO would be expected to form?
(Multiple Choice)
4.8/5  (42)
(42)
How many moles of aluminum are needed to make 9 moles of molecular hydrogen? Given the reaction:
2 Al + 6 HCl → 2 AlCl3 + 3H2
(Multiple Choice)
4.8/5  (41)
(41)
How many moles of NH3 can be produced by the reaction of 2.00 g of N2 with 3.00 g H2?
Reaction: N2(g)+ 3 H2(g)→ 2 NH3(g)
(Multiple Choice)
4.8/5  (34)
(34)
Starting with 156 g Li2O and 33.3 g H2O,decide which reactant is present in limiting quantities.
Given: Li2O + H2O → 2 LiOH
(Multiple Choice)
4.9/5  (43)
(43)
A sample of 8.5 g NH3 on oxidation produces 4.5 g of NO.Calculate the percent yield. Reaction: 4 NH3 + 5 O2 → 4 NO + 6 H2O
(Multiple Choice)
4.7/5  (40)
(40)
For the following reaction you have 8 grams of hydrogen and 2 grams of oxygen.
2H2 + O2 → 2H2O
The excess reactant is the oxygen.
(True/False)
4.7/5  (34)
(34)
A tricycle factory uses the following items to produce one tricycle: 3 tires,1 frame,and 2 pedals. If the factory has available 270 tires,90 frames,and 170 pedals,which item would limit the amount of complete tricycles that can be assembled?
(Multiple Choice)
4.9/5  (46)
(46)
Showing 101 - 115 of 115
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)