Exam 8: Quantities in Chemical Reactions
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases123 Questions
Exam 12: Liquids, solids, and Intermolecular Forces115 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry109 Questions
Exam 19: Biochemistry110 Questions
Select questions type
What is the excess reactant for the reaction below given that you start with 10.0 grams of Al and 19.0 grams of O2?
Reaction: 4Al + 3O2 → 2Al2O3
(Multiple Choice)
4.9/5  (32)
(32)
The limiting reactant is the reactant that produces the maximum amount of product.
(True/False)
4.8/5  (35)
(35)
Given the balanced equation CO2 + Si → SiO2 + C,if you were to react 1 mole of CO2 with 1 mole of Si,which statement is TRUE?
(Multiple Choice)
4.8/5  (29)
(29)
If the theoretical yield of the reaction below corresponds to 25.3 g and the percent yield of the reaction is known to be reproducibly 81.1%,calculate the actual yield.
Given: Li2O + H2O → 2 LiOH
(Multiple Choice)
4.9/5  (43)
(43)
The conversion factor for moles of carbon dioxide to mass of carbon dioxide is:
1 mole CO2 ≡ 44.01 g.
(True/False)
4.9/5  (41)
(41)
Global warming is due to the greenhouse gases preventing heat from Earth escaping into space.
(True/False)
4.9/5  (33)
(33)
Since heat must be supplied to melt ice,the melting of ice is an endothermic process and so has a positive enthalpy value.
(True/False)
4.8/5  (31)
(31)
Which is the excess reactant in the following reaction given that you start with 15.5 g of Na2S and 12.1 g CuSO4?
Reaction: Na2S + CuSO4 → Na2SO4 + CuS
(Multiple Choice)
4.8/5  (37)
(37)
How many moles of water are made from complete reaction of 2.2 moles of oxygen gas with hydrogen gas?
Given the reaction:
2H2 + O2 → 2H2O
(Multiple Choice)
4.8/5  (38)
(38)
What is the theoretical yield of waffles if you have 6 cups of flour,9 eggs and 2 tbs of oil? Given: 2 cups flour + 3 eggs + 1 tbs oil → 4 waffles
(Multiple Choice)
4.9/5  (39)
(39)
The theoretical yield of a reaction is 75.0 grams of product and the actual yield is 42.0g.What is the percent yield?
(Multiple Choice)
4.9/5  (34)
(34)
What is the limiting reactant for the following reaction given we have 2.6 moles of HCl and 1.4 moles of Ca(OH)2?
Reaction: 2HCl + Ca(OH)2 → 2H2O + CaCl2
(Multiple Choice)
4.9/5  (39)
(39)
Given that 4 NH3 + 5 O2 → 4 NO + 6 H2O,when 4.50 mol of H2O are formed,the amount of NO formed is:
(Multiple Choice)
4.7/5  (43)
(43)
What is the theoretical yield of a reaction if 25.0 grams of product were actually produced from a reaction that has a 88% yield?
(Multiple Choice)
4.8/5  (51)
(51)
How many grams of the excess reactant remain assuming the reaction goes to completion and that you start with 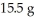 of Na2S and
of Na2S and 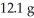 CuSO4?
Reaction: Na2S + CuSO4 → Na2SO4 + CuS
CuSO4?
Reaction: Na2S + CuSO4 → Na2SO4 + CuS
(Multiple Choice)
4.7/5  (33)
(33)
How many grams of NO2 are theoretically produced if we start with 1.20 moles of S and 9.90 moles of HNO3?
Reaction: S + 6HNO3 → H2SO4 + 6NO2 + 2H2O
(Multiple Choice)
5.0/5  (39)
(39)
Determine the theoretical yield of C when 3 units of A and 10 units of B are reacted in the following generic chemical equation: 2A + 5B → 4C.
(Multiple Choice)
4.8/5  (36)
(36)
How many moles of water are needed to react with 2.2 moles of Li2O?
Given: Li2O + H2O → 2 LiOH
(Multiple Choice)
4.8/5  (46)
(46)
If the theoretical yield of the reaction below corresponds to 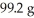 and the actual yield was 60.9 g,calculate the percent yield.
Given: Li2O + H2O → 2 LiOH
and the actual yield was 60.9 g,calculate the percent yield.
Given: Li2O + H2O → 2 LiOH
(Multiple Choice)
4.8/5  (31)
(31)
Showing 21 - 40 of 115
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)