Exam 8: Quantities in Chemical Reactions
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases123 Questions
Exam 12: Liquids, solids, and Intermolecular Forces115 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry109 Questions
Exam 19: Biochemistry110 Questions
Select questions type
How many waffles can be made from 1 dozen eggs,assuming you have enough of all other ingredients?
Given: 2 cups flour + 3 eggs + 1 tbs oil → 4 waffles
(Multiple Choice)
4.9/5  (33)
(33)
What is the theoretical yield of waffles if you have 5 cups of flour,9 eggs and 3 tbs of oil? Given: 2 cups flour + 3 eggs + 1 tbs oil → 4 waffles
(Multiple Choice)
5.0/5  (24)
(24)
Given the recipe: 2 cups flour + 1 egg + 3 oz blueberries → 4 muffins.
You can make 1 dozen muffins from 3 eggs.
(True/False)
4.9/5  (39)
(39)
How many moles of aluminum oxide are produced according to the reaction below given that you start with 10.0 grams of Al and 19.0 grams of O2?
Reaction: 4Al + 3O2 → 2Al2O3
(Multiple Choice)
4.9/5  (40)
(40)
How many grams of water are theoretically produced for the following reaction given we have 2.6 moles of HCl and 1.4 moles of Ca(OH)2?
Reaction: 2HCl + Ca(OH)2 → 2H2O + CaCl2
(Multiple Choice)
4.7/5  (38)
(38)
What is the limiting reactant for the reaction below given that you start with 10.0 grams of Al and 19.0 grams of O2?
Reaction: 4Al + 3O2 → 2Al2O3
(Multiple Choice)
4.9/5  (36)
(36)
Which is the limiting reactant in the following reaction given that you start with 15.5 g of Na2S and 12.1 g CuSO4?
Reaction: Na2S + CuSO4 → Na2SO4 + CuS
(Multiple Choice)
4.7/5  (44)
(44)
Consider the following generic chemical equation: 2A + 5B → C + 3D If you react 4 units of A with 10 units of B,which statement is TRUE?
(Multiple Choice)
4.9/5  (35)
(35)
How many grams of sodium metal are needed to make 29.3 grams of sodium chloride? Given the reaction:
2Na + Cl2 → 2NaCl
(Multiple Choice)
4.9/5  (29)
(29)
The reaction of one mole of nitrogen gas with three moles of hydrogen gas releases 92 kJ of thermal energy to the surroundings.Which of the following is TRUE?
(Multiple Choice)
4.7/5  (44)
(44)
Suppose two chemical reactions are linked together in a way that the O2 produced in the first reaction goes on to react completely with Mg to form MgO in the second reaction. Reaction one:
2 KClO3 → 3 O2 + 2 KCl
Reaction two: 2 Mg + O2 → 2 MgO
If you start with 4 moles of KClO3,how many moles of MgO could eventually form?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the below statements about global warming is FALSE?
(Multiple Choice)
4.8/5  (29)
(29)
The primary source for the rising carbon dioxide levels is respiration of the Earth's growing population.
(True/False)
4.7/5  (38)
(38)
The actual yield is the amount of product actually produced by a chemical reaction.
(True/False)
4.8/5  (36)
(36)
The enthalpy of reaction,△H rxn,is the amount of thermal energy that flows when a reaction occurs at constant temperature.
(True/False)
4.7/5  (49)
(49)
Before determining conversion factors,it is necessary to make sure the equation is properly balanced.
(True/False)
4.9/5  (30)
(30)
Hydrochloric acid reacts with barium hydroxide according to the equation: 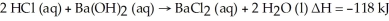 Calculate the heat (in kJ)associated with the complete reaction of 18.2 grams of HCl (aq).
Calculate the heat (in kJ)associated with the complete reaction of 18.2 grams of HCl (aq).
(Multiple Choice)
4.7/5  (40)
(40)
What is the limiting reactant for the reaction below given that you start with 2.50 grams C and 2.50 grams SiO2?
Reaction: C + SiO2 → SiC + O2
(Multiple Choice)
4.9/5  (34)
(34)
How many grams of water are made from the reaction of 16.0 grams of oxygen gas?
Given the reaction: 2H2 + O2 → 2H2O
(Multiple Choice)
4.8/5  (37)
(37)
Showing 61 - 80 of 115
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)