Exam 16: Mixtures
Exam 1: Patterns of Motion and Equilibrium126 Questions
Exam 2: Newtons Laws of Motion127 Questions
Exam 3: Momentum and Energy181 Questions
Exam 4: Gravity, Projectiles, and Satellites147 Questions
Exam 5: Fluid Mechanics148 Questions
Exam 6: Thermal Energy and Thermodynamics113 Questions
Exam 7: Heat Transfer and Change of Phase158 Questions
Exam 8: Static and Current Electricity178 Questions
Exam 9: Magnetism and Electromagnetic Induction130 Questions
Exam 10: Waves and Sound146 Questions
Exam 11: Light163 Questions
Exam 12: Atoms and the Periodic Table137 Questions
Exam 13: The Atomic Nucleus and Radioactivity127 Questions
Exam 14: Elements of Chemistry69 Questions
Exam 15: How Atoms Bond and Molecules Attract150 Questions
Exam 16: Mixtures141 Questions
Exam 17: How Chemicals React120 Questions
Exam 18: Two Classes of Chemical Reactions177 Questions
Exam 19: Organic Compounds96 Questions
Exam 20: Rocks and Minerals169 Questions
Exam 21: Plate Tectonics and Earths Interior181 Questions
Exam 22: Shaping Earths Surface180 Questions
Exam 23: Geologic Time - Reading the Rock Record167 Questions
Exam 24: The Oceans, Atmosphere, and Climatic Effects188 Questions
Exam 25: Driving Forces of Weather166 Questions
Exam 26: The Solar System108 Questions
Exam 27: Stars and Galaxies107 Questions
Exam 28: The Structure of Space and Time73 Questions
Exam 29: Prologue: The Nature of Science22 Questions
Select questions type
In a solution made from one teaspoon of sugar and one liter of water,which is the solute?
Free
(Multiple Choice)
4.7/5  (38)
(38)
Correct Answer:
A
How does the purchase and use of a home beverage carbonator help to minimize carbon dioxide emissions?
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
A
Which of the following solutions is the most dilute?
Free
(Multiple Choice)
4.7/5  (34)
(34)
Correct Answer:
E
How would you classify the following material? a cappuccino (with foam)
(Multiple Choice)
4.8/5  (37)
(37)
Water being purified using aluminum salts and a base is an example of
(Multiple Choice)
5.0/5  (39)
(39)
Half-frozen fruit punch is always sweeter than the same fruit punch completely melted because
(Multiple Choice)
4.8/5  (33)
(33)
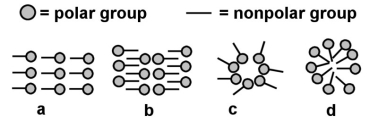 -Which of the above might best describe how soap behaves in a nonpolar solvent like turpentine?
-Which of the above might best describe how soap behaves in a nonpolar solvent like turpentine?
(Multiple Choice)
4.7/5  (32)
(32)
Based on atomic size,which would you expect to be more soluble in water: helium,He,or nitrogen, N2?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following might have the lowest solubility in water?
(Multiple Choice)
4.9/5  (26)
(26)
Phosphate ions, 3-,were once added to detergents to assist in cleaning.What function did they serve?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following statements best describes what is happening in a water softening unit?
(Multiple Choice)
4.7/5  (32)
(32)
How is most of the energy required for secondary wastewater treatment consumed?
(Multiple Choice)
4.8/5  (41)
(41)
What is the main difference between a soap and a detergent?
(Multiple Choice)
4.9/5  (32)
(32)
What is the special property of a semipermeable membrane that makes osmosis possible?
(Multiple Choice)
4.9/5  (40)
(40)
Showing 1 - 20 of 141
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)