Exam 17: How Chemicals React
Exam 1: Patterns of Motion and Equilibrium126 Questions
Exam 2: Newtons Laws of Motion127 Questions
Exam 3: Momentum and Energy181 Questions
Exam 4: Gravity, Projectiles, and Satellites147 Questions
Exam 5: Fluid Mechanics148 Questions
Exam 6: Thermal Energy and Thermodynamics113 Questions
Exam 7: Heat Transfer and Change of Phase158 Questions
Exam 8: Static and Current Electricity178 Questions
Exam 9: Magnetism and Electromagnetic Induction130 Questions
Exam 10: Waves and Sound146 Questions
Exam 11: Light163 Questions
Exam 12: Atoms and the Periodic Table137 Questions
Exam 13: The Atomic Nucleus and Radioactivity127 Questions
Exam 14: Elements of Chemistry69 Questions
Exam 15: How Atoms Bond and Molecules Attract150 Questions
Exam 16: Mixtures141 Questions
Exam 17: How Chemicals React120 Questions
Exam 18: Two Classes of Chemical Reactions177 Questions
Exam 19: Organic Compounds96 Questions
Exam 20: Rocks and Minerals169 Questions
Exam 21: Plate Tectonics and Earths Interior181 Questions
Exam 22: Shaping Earths Surface180 Questions
Exam 23: Geologic Time - Reading the Rock Record167 Questions
Exam 24: The Oceans, Atmosphere, and Climatic Effects188 Questions
Exam 25: Driving Forces of Weather166 Questions
Exam 26: The Solar System108 Questions
Exam 27: Stars and Galaxies107 Questions
Exam 28: The Structure of Space and Time73 Questions
Exam 29: Prologue: The Nature of Science22 Questions
Select questions type
Which of the following statements about catalysts is NOT true?
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
E
Why might increasing the temperature alter the rate of a chemical reaction?
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
A
What is the main difficulty in trying to eliminate chlorinated fluorocarbons from the atmosphere?
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
A
Given the following generic chemical reaction,which is the product? X → Y
(Multiple Choice)
4.9/5  (36)
(36)
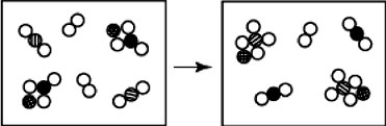 -How many diatomic molecules are represented in the illustration above?
-How many diatomic molecules are represented in the illustration above?
(Multiple Choice)
4.8/5  (36)
(36)
Which is higher in an endothermic reaction: the potential energy of the reactants or the potential energy of the products?
(Multiple Choice)
4.8/5  (37)
(37)
If the relative mass of a pingpong ball is 1/20 that of a golf ball,how many pingpong balls would you need to equal the mass of two golf balls?
(Multiple Choice)
4.9/5  (36)
(36)
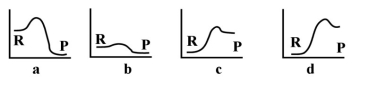 -Given that the above energy profiles have the same scale,which of the reactions would require the most energy?
-Given that the above energy profiles have the same scale,which of the reactions would require the most energy?
(Multiple Choice)
4.8/5  (34)
(34)
 -Which equation best describes the reaction represented in the illustration above?
-Which equation best describes the reaction represented in the illustration above?
(Multiple Choice)
4.9/5  (35)
(35)
How many grams of water, O,and propene, ,can be formed from the reaction of 6.0 g of 2-propanol, O? O ? + O 2-Propanol Propene Water
(Multiple Choice)
4.7/5  (35)
(35)
What can you deduce about the activation energy of a reaction that takes billions of years to go to completion? How about a reaction that takes only fractions of a second?
(Multiple Choice)
4.8/5  (42)
(42)
Are the chemical reactions that take place in a disposable battery exothermic or endothermic? Is the reaction going on in a rechargeable battery while it is recharging exothermic or endothermic?
(Multiple Choice)
4.9/5  (35)
(35)
Some reactions are more sluggish than others.To speed up these reactions and save energy a(n)________ is sometimes added.
(Multiple Choice)
4.7/5  (30)
(30)
According to the following balanced chemical equation,if you want to generate two moles of H2O,how many molecules of O2 do you need? 2 H2 + O2 → 2 H2O
(Multiple Choice)
4.9/5  (27)
(27)
Balance the following equation. ________ NO → ________ N2O + ________ NO2
(Multiple Choice)
4.7/5  (40)
(40)
If the relative mass of a hydrogen atom is 1/4 that of a helium atom,how many helium atoms would you need to equal the mass of 200 hydrogen atoms?
(Multiple Choice)
4.8/5  (36)
(36)
Why does a glowing splint of wood burn only slowly in air,but rapidly in a burst of flames when placed in pure oxygen?
(Multiple Choice)
4.9/5  (28)
(28)
Why does iodine, (s),spontaneously sublime at room temperature?
(Multiple Choice)
4.7/5  (30)
(30)
Showing 1 - 20 of 120
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)