Exam 18: Two Classes of Chemical Reactions
Exam 1: Patterns of Motion and Equilibrium126 Questions
Exam 2: Newtons Laws of Motion127 Questions
Exam 3: Momentum and Energy181 Questions
Exam 4: Gravity, Projectiles, and Satellites147 Questions
Exam 5: Fluid Mechanics148 Questions
Exam 6: Thermal Energy and Thermodynamics113 Questions
Exam 7: Heat Transfer and Change of Phase158 Questions
Exam 8: Static and Current Electricity178 Questions
Exam 9: Magnetism and Electromagnetic Induction130 Questions
Exam 10: Waves and Sound146 Questions
Exam 11: Light163 Questions
Exam 12: Atoms and the Periodic Table137 Questions
Exam 13: The Atomic Nucleus and Radioactivity127 Questions
Exam 14: Elements of Chemistry69 Questions
Exam 15: How Atoms Bond and Molecules Attract150 Questions
Exam 16: Mixtures141 Questions
Exam 17: How Chemicals React120 Questions
Exam 18: Two Classes of Chemical Reactions177 Questions
Exam 19: Organic Compounds96 Questions
Exam 20: Rocks and Minerals169 Questions
Exam 21: Plate Tectonics and Earths Interior181 Questions
Exam 22: Shaping Earths Surface180 Questions
Exam 23: Geologic Time - Reading the Rock Record167 Questions
Exam 24: The Oceans, Atmosphere, and Climatic Effects188 Questions
Exam 25: Driving Forces of Weather166 Questions
Exam 26: The Solar System108 Questions
Exam 27: Stars and Galaxies107 Questions
Exam 28: The Structure of Space and Time73 Questions
Exam 29: Prologue: The Nature of Science22 Questions
Select questions type
Chemical equations need to be balanced not only in terms of the number of atoms,but also by the charge.In other words,just as there should be the same number of atoms before and after the arrow of an equation,there should be the same charge.What set of coefficients is necessary to balance the following chemical equation? ________ + ________ Ag ? ________ Sn + ________ Ag?
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
A
Which of the following solutions is the most acidic?
Free
(Multiple Choice)
4.9/5  (30)
(30)
Correct Answer:
C
What is the hydroxide ion concentration in an aqueous solution where the pH = 5?
Free
(Multiple Choice)
4.9/5  (43)
(43)
Correct Answer:
C
What element is oxidized in the following equation and what element is reduced? + 2 Ag ? Sn + 2 Ag?
(Multiple Choice)
5.0/5  (35)
(35)
What would the concentration of OH- be if the concentration of H3O+ was 1 × 10-8M? [H3O+] × [OH-] = Kw = 1 × 10-14
(Multiple Choice)
4.9/5  (36)
(36)
In one type of fuel cell the following oxidation-reduction reactions are taking place: 2 H2 + O2 → 2 H2O
What is the fuel?
(Multiple Choice)
4.9/5  (33)
(33)
According to the following reaction,which molecule is acting as a base? H2O + H2SO4 → H3O+ + HSO4-
(Multiple Choice)
4.9/5  (37)
(37)
In a battery,the following two oxidation-reduction reactions are taking place: rxn A: Zn → Zn+2 + 2e-
Rxn B: 2 NH4+ + 2e- → 2 NH3 + H2
Which reaction is taking place at the cathode?
(Multiple Choice)
4.7/5  (29)
(29)
A chemical equation for the combustion of propane,C3H8,is shown below.Through this reaction is the carbon oxidized or reduced? + 5 ? 3 + 4 O
(Multiple Choice)
4.8/5  (39)
(39)
When the hydronium ion concentration equals 10 moles per liter,what is the pH of the solution? Is the solution acidic or basic?
(Multiple Choice)
4.8/5  (32)
(32)
What does the value of say about the extent to which water molecule react with themselves?
(Multiple Choice)
4.8/5  (43)
(43)
Along with the pH scale,there is the pOH scale,which indicates the level of "basicity" in a solution.Accordingly,pOH = -log[OH-].What is the sum of the pH and the pOH of a solution always equal to?
(Multiple Choice)
4.7/5  (37)
(37)
Which of the following statements best describes a cathode?
(Multiple Choice)
4.9/5  (22)
(22)
Which of the following statements about strong and weak acids is not true?
(Multiple Choice)
4.8/5  (35)
(35)
If you had a 1 M solution of a strong acid what would be its pH?
(Multiple Choice)
4.8/5  (37)
(37)
For the following reaction,identify whether the compound in bold is behaving as an acid or a base. H3PO4 + H2O ⇌ H2PO4- + H3O+
(Multiple Choice)
4.7/5  (33)
(33)
Rust has a tendency to form when iron in contact with water reacts according to the following reaction: 4 Fe + 3 O2 → 2 Fe2O3
What is happening at the cathodic region?
(Multiple Choice)
4.7/5  (30)
(30)
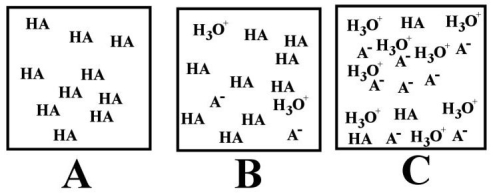 -Which of the above images best depicts a water solution of a weak acid (represented by HA)?
-Which of the above images best depicts a water solution of a weak acid (represented by HA)?
(Multiple Choice)
4.7/5  (21)
(21)
Iron atoms have a greater tendency to oxidize than do copper atoms.Is this good news or bad news for a home in which much of the plumbing consists of iron and copper pipes connected together? Explain.
(Multiple Choice)
4.9/5  (34)
(34)
What is the relationship between the hydroxide ion and a water molecule?
(Multiple Choice)
4.8/5  (46)
(46)
Showing 1 - 20 of 177
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)