Exam 10: Chemical Reactions
Exam 1: What Is Science30 Questions
Exam 2: Motion30 Questions
Exam 3: Energy30 Questions
Exam 4: Heat and Temperature30 Questions
Exam 5: Wave Motions and Sound30 Questions
Exam 6: Electricity30 Questions
Exam 7: Light35 Questions
Exam 8: Atoms and Periodic Properties30 Questions
Exam 9: Chemical Bonds30 Questions
Exam 10: Chemical Reactions30 Questions
Exam 11: Water and Solutions30 Questions
Exam 12: Organic Chemistry30 Questions
Exam 13: Nuclear Reactions30 Questions
Exam 14: The Universe30 Questions
Exam 15: The Solar System30 Questions
Exam 16: Earth in Space30 Questions
Exam 17: Rocks and Minerals30 Questions
Exam 18: Plate Tectonics30 Questions
Exam 19: Building Earths Surface30 Questions
Exam 20: Shaping Earths Surface30 Questions
Exam 21: Geologic Time30 Questions
Exam 22: The Atmosphere of Earth29 Questions
Exam 23: Weather and Climate30 Questions
Exam 24: Earths Waters30 Questions
Select questions type
The reaction between water solutions of sodium chloride and silver nitrate produces a precipitate: NaCl(aq) + AgNO3(aq) NaNO3(aq) + AgCl(s).This is an example of
(Multiple Choice)
4.7/5  (29)
(29)
Consider the chemical equation: 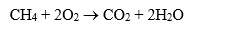 -What is the number of atoms on each side of the equation?
-What is the number of atoms on each side of the equation?
(Multiple Choice)
4.8/5  (31)
(31)
When the equation __Li + __O2 __Li2O is correctly balanced, what is the sum of the coefficients?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following is a sign of an ion exchange reaction?
(Multiple Choice)
4.7/5  (33)
(33)
How many molecules are in a 8.0-g sample of oxygen gas, O2?
(Multiple Choice)
4.8/5  (39)
(39)
The equation 2 C2H5OH + __O2 4 CO2 + 6 H2O is balanced by making the coefficient of oxygen
(Multiple Choice)
4.7/5  (30)
(30)
When balancing a chemical equation, the number of H atoms in 2 CH4 is eight.
(True/False)
4.8/5  (34)
(34)
The sum of the coefficients must be the same on both sides of a chemical equation.
(True/False)
4.9/5  (35)
(35)
What is the mass percent of sodium in washing soda, Na2CO3?
(Multiple Choice)
4.9/5  (42)
(42)
Showing 21 - 30 of 30
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)