Exam 7: Quantum Theory and the Electronic Structure of Atoms
Exam 1: Chemistry: The Study of Change153 Questions
Exam 2: Atoms, Molecules, and Ions141 Questions
Exam 3: Mass Relationships in Chemical Reactions168 Questions
Exam 4: Reactions in Aqueous Solution161 Questions
Exam 5: Gases109 Questions
Exam 6: Thermo-Chemistry111 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms115 Questions
Exam 8: Periodic Relationships Among the Elements119 Questions
Exam 9: Chemical Bonding I: Basic Concepts118 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals122 Questions
Exam 11: Intermolecular Forces and Liquids and Solids140 Questions
Exam 12: Physical Properties of Solutions109 Questions
Exam 13: Chemical Kinetics114 Questions
Exam 14: Chemical Equilibrium100 Questions
Exam 15: Acids and Bases163 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria110 Questions
Exam 17: Chemistry in the Atmosphere41 Questions
Exam 18: Entropy, Free Energy, and Equilibrium112 Questions
Exam 19: Electrochemistry138 Questions
Exam 20: Metallurgy and the Chemistry of Metals58 Questions
Exam 21: Nonmetallic Elements and Their Compounds41 Questions
Exam 22: Transition Metal Chemistry and Coordination Compounds80 Questions
Exam 23: Nuclear Chemistry112 Questions
Exam 24: Organic Chemistry57 Questions
Exam 25: Synthetic and Natural Organic Polymers42 Questions
Select questions type
The electron configuration of a ground-state vanadium atom is
(Multiple Choice)
4.8/5  (38)
(38)
Which one of the following sets of quantum numbers is not possible? 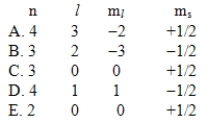
(Short Answer)
4.9/5  (36)
(36)
Calculate the frequency of visible light having a wavelength of 686 nm.
(Multiple Choice)
4.9/5  (35)
(35)
A ground-state atom of manganese has ___ unpaired electrons and is _____.
(Multiple Choice)
5.0/5  (34)
(34)
Which of the following is the electron configuration of an excited state of an iron atom?
(Multiple Choice)
4.8/5  (28)
(28)
What is the wavelength of radiation that has a frequency of 2.10 *1014 s -1?
(Multiple Choice)
4.8/5  (33)
(33)
What is the wavelength, in meters, of an alpha particle with a kinetic energy of 8.0 * 10-13 J.(mass of an alpha particle = 4.00150 amu; 1 amu = 1.67 * 10-27 kg)
(Short Answer)
4.8/5  (40)
(40)
The scattering of helium atoms by a solid can be used to gain information about the lattice of atoms, provided the wavelength of the helium atoms is comparable to the dimensions of the lattice.What is the wavelength, in picometers, of a helium-4 atom with a kinetic energy of 0.10 eV.(mass of 4He = 4.0026 amu; 1 amu = 1.67 * 10-24 g; 1 eV = 1.602 * 10-19 J; h = 6.626 * 10-34 J.s)
(Short Answer)
4.8/5  (38)
(38)
The ground-state electron configuration for an atom of indium is
(Multiple Choice)
4.9/5  (27)
(27)
How many orbitals are allowed in a subshell if the angular momentum quantum number for electrons in that subshell is 3?
(Multiple Choice)
4.8/5  (38)
(38)
The electron in a hydrogen atom falls from an excited energy level to the ground state in two steps, causing the emission of photons with wavelengths of 2624 and 97.2 nm. What is the quantum number of the initial excited energy level from which the electron falls?
(Multiple Choice)
4.8/5  (32)
(32)
If a hydrogen atom and a helium atom have the same kinetic energy,
(Multiple Choice)
4.8/5  (42)
(42)
What is the energy in joules of one photon of microwave radiation with a wavelength 0.122 m? (c = 2.9979 * 108 m/s; h = 6.626 * 10-34 J0s)
(Multiple Choice)
4.8/5  (45)
(45)
Breaking the oxygen-oxygen bond in hydrogen peroxide requires 210 kJ/mol. What is the longest wavelength of light that can cause this bond to be broken?
(Multiple Choice)
4.8/5  (43)
(43)
Calculate the frequency of the light emitted by a hydrogen atom during a transition of its electron from the n = 4 to the n = 1 principal energy level. Recall that for hydrogen En = -2.18 *10 -18 J(1/n2)
(Multiple Choice)
4.9/5  (27)
(27)
The bonds of oxygen molecules are broken by sunlight.The minimum energy required to break the oxygen-oxygen bond is 495 kJ/mol. What is the wavelength of sunlight that can cause this bond breakage?
(Short Answer)
4.8/5  (35)
(35)
How many electrons in a ground-state tellurium atom are in orbitals labeled by l = 1?
(Multiple Choice)
4.9/5  (43)
(43)
If we take away two electrons from the outer shell of calcium, it would have the same electron configuration as what element?
(Short Answer)
4.7/5  (35)
(35)
Calculate the wavelength of a neutron that has a velocity of 250 cm/s. (The mass of a neutron = 1.675 * 10-24 g; h = 6.626 * 10-34 J.s)
(Multiple Choice)
4.8/5  (32)
(32)
Showing 81 - 100 of 115
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)