Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals
Exam 1: Chemistry: The Study of Change153 Questions
Exam 2: Atoms, Molecules, and Ions141 Questions
Exam 3: Mass Relationships in Chemical Reactions168 Questions
Exam 4: Reactions in Aqueous Solution161 Questions
Exam 5: Gases109 Questions
Exam 6: Thermo-Chemistry111 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms115 Questions
Exam 8: Periodic Relationships Among the Elements119 Questions
Exam 9: Chemical Bonding I: Basic Concepts118 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals122 Questions
Exam 11: Intermolecular Forces and Liquids and Solids140 Questions
Exam 12: Physical Properties of Solutions109 Questions
Exam 13: Chemical Kinetics114 Questions
Exam 14: Chemical Equilibrium100 Questions
Exam 15: Acids and Bases163 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria110 Questions
Exam 17: Chemistry in the Atmosphere41 Questions
Exam 18: Entropy, Free Energy, and Equilibrium112 Questions
Exam 19: Electrochemistry138 Questions
Exam 20: Metallurgy and the Chemistry of Metals58 Questions
Exam 21: Nonmetallic Elements and Their Compounds41 Questions
Exam 22: Transition Metal Chemistry and Coordination Compounds80 Questions
Exam 23: Nuclear Chemistry112 Questions
Exam 24: Organic Chemistry57 Questions
Exam 25: Synthetic and Natural Organic Polymers42 Questions
Select questions type
According to the VSEPR theory, the molecular geometry of boron trichloride is
(Multiple Choice)
4.8/5  (34)
(34)
What is the hybridization of the As atom in the AsF5 molecule?
(Multiple Choice)
4.9/5  (43)
(43)
Indicate the type of hybrid orbitals used by the central atom in TeF4.
(Multiple Choice)
4.8/5  (37)
(37)
N,N-diethyl-m-tolumide (DEET)is the active ingredient in many mosquito repellents. 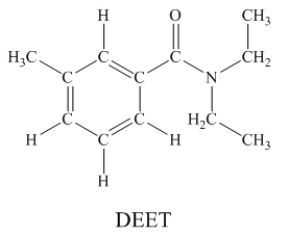 How many sigma bonds and pi bonds are contained in a DEET molecule?
How many sigma bonds and pi bonds are contained in a DEET molecule?
(Short Answer)
4.8/5  (37)
(37)
Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever. 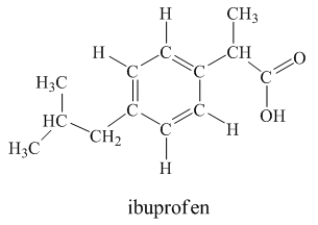 How many sigma bonds and pi bonds are contained in a ibuprofen molecule?
How many sigma bonds and pi bonds are contained in a ibuprofen molecule?
(Short Answer)
4.9/5  (44)
(44)
Which of the following species has the largest dipole moment (i.e., is the most polar)?
(Multiple Choice)
4.9/5  (40)
(40)
Predict the molecular geometry and polarity of the SO2 molecule.
(Multiple Choice)
4.8/5  (35)
(35)
Use VSEPR theory to explain why the water molecule is bent, rather than linear.
(Essay)
4.9/5  (28)
(28)
N,N-diethyl-m-tolumide (DEET)is the active ingredient in many mosquito repellents.What is the hybridization state of the nitrogen atom in the structure of DEET shown below? 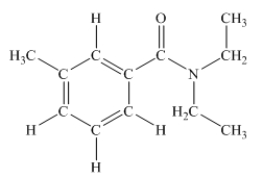
(Multiple Choice)
4.8/5  (42)
(42)
Use VSEPR theory to predict the molecular geometry of SF4 (sulfur tetrafluoride).
(Short Answer)
4.7/5  (40)
(40)
If a triatomic molecule is linear, then the hybridization of the central atom will be
(Multiple Choice)
4.8/5  (39)
(39)
Indicate the type of hybrid orbitals used by the central atom in SF6.
(Multiple Choice)
4.9/5  (43)
(43)
Showing 101 - 120 of 122
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)