Exam 11: Intermolecular Forces and Liquids and Solids
Exam 1: Chemistry: The Study of Change153 Questions
Exam 2: Atoms, Molecules, and Ions141 Questions
Exam 3: Mass Relationships in Chemical Reactions168 Questions
Exam 4: Reactions in Aqueous Solution161 Questions
Exam 5: Gases109 Questions
Exam 6: Thermo-Chemistry111 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms115 Questions
Exam 8: Periodic Relationships Among the Elements119 Questions
Exam 9: Chemical Bonding I: Basic Concepts118 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals122 Questions
Exam 11: Intermolecular Forces and Liquids and Solids140 Questions
Exam 12: Physical Properties of Solutions109 Questions
Exam 13: Chemical Kinetics114 Questions
Exam 14: Chemical Equilibrium100 Questions
Exam 15: Acids and Bases163 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria110 Questions
Exam 17: Chemistry in the Atmosphere41 Questions
Exam 18: Entropy, Free Energy, and Equilibrium112 Questions
Exam 19: Electrochemistry138 Questions
Exam 20: Metallurgy and the Chemistry of Metals58 Questions
Exam 21: Nonmetallic Elements and Their Compounds41 Questions
Exam 22: Transition Metal Chemistry and Coordination Compounds80 Questions
Exam 23: Nuclear Chemistry112 Questions
Exam 24: Organic Chemistry57 Questions
Exam 25: Synthetic and Natural Organic Polymers42 Questions
Select questions type
Helium atoms do not combine to form He2 molecules, yet He atoms do attract one another weakly through
(Multiple Choice)
4.8/5  (39)
(39)
Use the following data to determine the molar heat of vaporization of chlorine. 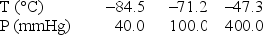
(Multiple Choice)
4.9/5  (36)
(36)
Osmium tetroxide, OsO4, is a soft crystal that melts at 40°C.The liquid does not conduct electricity.What kind of crystal is this?
(Short Answer)
4.8/5  (37)
(37)
Copper crystallizes in a face-centered cubic unit cell. The density of copper is 8.94 g/cm3.Calculate the length of the edge of the unit cell in pm.
(Short Answer)
4.8/5  (34)
(34)
Suppose the atoms in a two-dimensional crystal have the following arrangement: 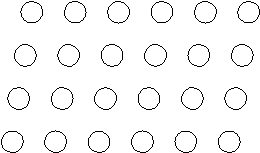 On the drawing above, sketch the unit cell of this crystal.
On the drawing above, sketch the unit cell of this crystal.
(Essay)
4.8/5  (35)
(35)
Which one of the following crystallizes in a metallic lattice?
(Multiple Choice)
4.9/5  (34)
(34)
Calculate the amount of heat that must be absorbed by 10.0 g of ice at -20°C to convert it to liquid water at 60.0°C. Given: specific heat (ice)= 2.1 J/g·°C; specific heat (water)= 4.18 J/g·°C; Hfus = 6.0 kJ/mol.
(Multiple Choice)
4.8/5  (49)
(49)
Platinum has a face-centered cubic crystal structure and a density of 21.5 g/cm3. What is the radius of the platinum atom?
(Multiple Choice)
4.9/5  (39)
(39)
Which one of the following substances crystallizes as a covalent crystal?
(Multiple Choice)
4.9/5  (33)
(33)
Potassium crystallizes in a body-centered cubic lattice.How many atoms are there per unit cell?
(Multiple Choice)
4.8/5  (31)
(31)
How much energy (heat)is required to convert 52.0 g of ice at -10.0°C to steam at 100°C? 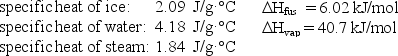
(Multiple Choice)
4.7/5  (33)
(33)
Of the given pair of compounds, which would have the higher boiling point?
CH3Cl or CH4
(Short Answer)
4.8/5  (43)
(43)
Which is expected to have a higher boiling point, C5H12 or C(CH3)4?
(Short Answer)
4.9/5  (31)
(31)
Indicate all the types of intermolecular forces of attraction in CH2O(g).
(Short Answer)
4.7/5  (48)
(48)
Each of the following substances is a liquid at -50°C. Place these liquids in order of increasing vapor pressure: dimethyl ether (CH3OCH3), propane (C3H8), and ethanol (CH3CH2OH).
(Multiple Choice)
4.8/5  (33)
(33)
Which one of the following substances should exhibit hydrogen bonding in the liquid state?
(Multiple Choice)
4.9/5  (42)
(42)
Butter melts over a range of temperature, rather than with a sharp melting point. Butter is classified as a/an
(Multiple Choice)
4.7/5  (35)
(35)
Which of the following liquids would have the lowest viscosity at 25°C? 
(Short Answer)
4.8/5  (31)
(31)
Given that the heat of vaporization of mercury is 59.0 kJ/mol and the vapor pressure of mercury is 0.0017 torr at 25°C, calculate the normal boiling point of mercury.
(Short Answer)
4.8/5  (30)
(30)
The structural form of the element Ge closely resembles the structure of
(Multiple Choice)
4.8/5  (44)
(44)
Showing 21 - 40 of 140
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)