Exam 11: Intermolecular Forces and Liquids and Solids
Exam 1: Chemistry: The Study of Change153 Questions
Exam 2: Atoms, Molecules, and Ions141 Questions
Exam 3: Mass Relationships in Chemical Reactions168 Questions
Exam 4: Reactions in Aqueous Solution161 Questions
Exam 5: Gases109 Questions
Exam 6: Thermo-Chemistry111 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms115 Questions
Exam 8: Periodic Relationships Among the Elements119 Questions
Exam 9: Chemical Bonding I: Basic Concepts118 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals122 Questions
Exam 11: Intermolecular Forces and Liquids and Solids140 Questions
Exam 12: Physical Properties of Solutions109 Questions
Exam 13: Chemical Kinetics114 Questions
Exam 14: Chemical Equilibrium100 Questions
Exam 15: Acids and Bases163 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria110 Questions
Exam 17: Chemistry in the Atmosphere41 Questions
Exam 18: Entropy, Free Energy, and Equilibrium112 Questions
Exam 19: Electrochemistry138 Questions
Exam 20: Metallurgy and the Chemistry of Metals58 Questions
Exam 21: Nonmetallic Elements and Their Compounds41 Questions
Exam 22: Transition Metal Chemistry and Coordination Compounds80 Questions
Exam 23: Nuclear Chemistry112 Questions
Exam 24: Organic Chemistry57 Questions
Exam 25: Synthetic and Natural Organic Polymers42 Questions
Select questions type
Which of the responses includes all of the following that can form hydrogen bonds with water molecules? (1)Na+ (2)CH3COOH (3)C2H6 (4)CH3NH2
(Multiple Choice)
4.8/5  (38)
(38)
Which liquid is expected to have the larger surface tension at a given temperature, CCl4 or H2O? Briefly explain.
(Essay)
4.9/5  (39)
(39)
Which of the following substances should have the highest boiling point?
(Multiple Choice)
4.8/5  (39)
(39)
Use the graph of vapor pressure to determine the normal boiling point of CHCl3. 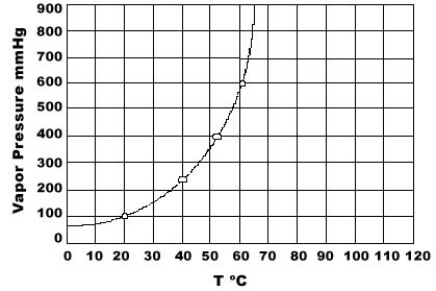
(Multiple Choice)
4.8/5  (36)
(36)
Suppose the atoms in a two-dimensional crystal have the following arrangement: 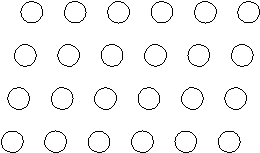 What is the coordination number of each atom in this crystal?
What is the coordination number of each atom in this crystal?
(Short Answer)
4.8/5  (36)
(36)
The molar enthalpy of vaporization of hexane (C6H14)is 28.9 kJ/mol, and its normal boiling point is 68.73°C. What is the vapor pressure of hexane at 25°C?
(Multiple Choice)
4.9/5  (41)
(41)
Of the pair of compounds given, which would have the stronger intermolecular forces of attraction?
CH4 or CH3OH
(Short Answer)
4.7/5  (38)
(38)
Indicate all the types of intermolecular forces of attraction in SF4(g).
(Short Answer)
4.7/5  (30)
(30)
Of the pair of compounds given, which would have the stronger intermolecular forces of attraction?
NH3 or PH3
(Short Answer)
4.7/5  (30)
(30)
Silver metal crystallizes in a face-centered cubic lattice with L as the length of one edge of the unit cube. The center-to-center distance between nearest silver atoms is
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following characteristics indicates the presence of weak intermolecular forces in a liquid?
(Multiple Choice)
4.9/5  (36)
(36)
Octane, C8H18, boils at 125°C as compared to water, which boils at 100°C. This information suggests that the dispersion forces in nonpolar octane molecules are stronger than dispersion forces and hydrogen bonding in water.
(True/False)
4.8/5  (36)
(36)
The vapor pressure of a liquid in a closed container depends upon
(Multiple Choice)
4.9/5  (38)
(38)
The normal boiling point of bromine is 58.8°C. Given that the vapor pressure of bromine is 75.0 torr at 2.5°C, calculate the molar enthalpy of vaporization of bromine.
(Multiple Choice)
4.7/5  (39)
(39)
The normal boiling point of methanol (CH3OH)is 64.6°C. Given that the vapor pressure of methanol is 75.0 torr at 15.2°C, calculate the molar enthalpy of vaporization of methanol.
(Multiple Choice)
4.8/5  (38)
(38)
Given the following liquids and their boiling points, which has the highest vapor pressure at its normal boiling point?
(Multiple Choice)
4.8/5  (29)
(29)
Of the given pair of compounds, which would have the higher boiling point?
H2Se or H2O
(Short Answer)
4.9/5  (33)
(33)
Of the pair of compounds given, which would have the stronger intermolecular forces of attraction?
SF4 or C10H22
(Short Answer)
4.9/5  (40)
(40)
Showing 41 - 60 of 140
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)