Exam 1: Introduction: Matter and Measurement
Exam 1: Introduction: Matter and Measurement163 Questions
Exam 2: Atoms, Molecules, and Ions250 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations178 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry180 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding147 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry114 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
The correct answer (reported to the proper number of significant figures)to the following is ________. (12.67 + 19.2)(3.99)/ (1.36 + 11.366)= ________
Free
(Multiple Choice)
4.7/5  (36)
(36)
Correct Answer:
D
What decimal power does the abbreviation d represent?
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
D
The quantity 1.0 mg/cm2 is the same as 1.0 × ________ kg/m2.
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
D
The density of mercury is 13.6 g/cm3. The density of mercury is ________ kg/m3.
(Multiple Choice)
4.8/5  (39)
(39)
The distance from the Earth to the Moon is approximately 240,000 miles. If a rocket travels at a speed of 7.50 km/sec, it will take ________ days to travel between the Earth and Moon.
(Multiple Choice)
4.8/5  (31)
(31)
In which one of the following numbers are none of the zeros significant?
(Multiple Choice)
4.9/5  (38)
(38)
One edge of a cube is measured and found to be 13 cm. The volume of the cube in m3 is ________.
(Multiple Choice)
4.8/5  (45)
(45)
A scientific theory is a concise statement or an equation that summarizes a broad variety of observations.
(True/False)
4.9/5  (29)
(29)
The length of the side of a cube (in cm)having a volume of 44.4 L is ________.
(Multiple Choice)
4.9/5  (36)
(36)
There are ________ significant figures in the answer to the following computation: 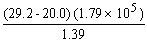
(Multiple Choice)
4.8/5  (23)
(23)
The density of air under ordinary conditions at 25 °C is 1.19 g/L. How many kilograms of air are in a room that measures 9.0 ft × 11.0 ft and has a 10.0 ft ceiling?
(Multiple Choice)
4.9/5  (46)
(46)
You have to calculate the mass of a 30.0 mL liquid sample with density of 1.52 g/mL, but you have forgotten the formula. Which way of reasoning would help you in finding the correct mass?
(Multiple Choice)
4.9/5  (33)
(33)
In which one of the following numbers are all of the zeros significant?
(Multiple Choice)
4.9/5  (35)
(35)
Showing 1 - 20 of 163
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)