Exam 1: Introduction: Matter and Measurement
Exam 1: Introduction: Matter and Measurement163 Questions
Exam 2: Atoms, Molecules, and Ions250 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations178 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry180 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding147 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry114 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
If an object is accelerating at a rate of 25 m/s2, how long (in seconds)will it take to reach a speed of 550 m/s? (Assume an initial velocity of zero.)
(Multiple Choice)
5.0/5  (32)
(32)
What is the physical state in which matter has no specific shape but does have a specific volume?
(Multiple Choice)
4.8/5  (32)
(32)
A cube of an unknown metal measures 0.250 cm on one side. The mass of the cube is 0.095 g. Which of the following is most likely the unknown metal? 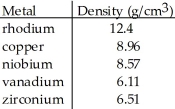
(Multiple Choice)
4.8/5  (47)
(47)
Momentum is defined as the product of mass and velocity. The SI unit for momentum is ________.
(Multiple Choice)
4.9/5  (32)
(32)
The density of lead is 11.4 g/cm3. The mass of a lead ball with a radius of 0.50 mm is ________ g. (π = 3.1416; Vsphere = 4πr3/3)
(Multiple Choice)
4.9/5  (34)
(34)
The width, length, and height of a large, custom-made shipping crate are 1.12 m, 1.25 m, and 0.83 m, respectively. The volume of the box using the correct number of significant figures is ________ m3.
(Multiple Choice)
5.0/5  (39)
(39)
The density of silver is 10.5 g/ cm3. A piece of silver that occupies a volume of 9.60 cm3 would have a mass of ________ g.
(Multiple Choice)
4.8/5  (29)
(29)
The correct result (indicating the proper number of significant figures)of the following addition is ________. 12
1.2
0.12
+ 0.012
(Multiple Choice)
5.0/5  (27)
(27)
The initial or tentative explanation of an observation is called a(n)________.
(Multiple Choice)
4.9/5  (34)
(34)
The unit of force in the English measurement system is 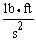 . The SI unit of force is the Newton, which is ________ in base SI units.
. The SI unit of force is the Newton, which is ________ in base SI units.
(Multiple Choice)
4.7/5  (39)
(39)
If matter is uniform throughout and cannot be separated into other substances by physical processes, but can be decomposed into other substances by chemical processes, it is called a(n)________.
(Multiple Choice)
4.8/5  (26)
(26)
Mass and volume are often referred to as ________ properties of substances.
(Short Answer)
4.9/5  (31)
(31)
An object measuring 0.4500 kilograms will have a mass of ________ grams.
(Multiple Choice)
4.8/5  (29)
(29)
Which of the following has the same number of significant figures as the number 0.0050?
(Multiple Choice)
4.7/5  (28)
(28)
Showing 21 - 40 of 163
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)