Exam 1: Introduction: Matter and Measurement
Exam 1: Introduction: Matter and Measurement163 Questions
Exam 2: Atoms, Molecules, and Ions250 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations178 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry180 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding147 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry114 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
The density (in g/cm3)of a gold nugget that has a volume of 1.68 cm3 and a mass of 32.4 g is ________.
(Multiple Choice)
4.8/5  (35)
(35)
An object measuring 0.76 decimeters will have a length of ________ centimeters.
(Multiple Choice)
4.9/5  (34)
(34)
You have to calculate the volume of a gas sample with mass of 1.000 × 103 g and density of 1.027 g/L, but you have forgotten the formula. Which way of reasoning would help you in finding the correct mass?
(Multiple Choice)
4.9/5  (41)
(41)
There should be ________ significant figures in the answer to the following computation. 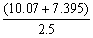
(Multiple Choice)
4.8/5  (31)
(31)
A 4.369 g sample of metal is placed in a flask. Water is added to the flask and the total volume in the flask is read to be 126.4 ml. The mass of the water, flask, and metal is 268.5 g. If the mass of the flask is 139.3 g and the density of water is 1.000 g/mL, the density of the solid is ________ g/cm3.
(Multiple Choice)
4.9/5  (28)
(28)
Which one of the following elements has a symbol that is not derived from its foreign name?
(Multiple Choice)
4.8/5  (32)
(32)
Osmium has a density of 22.6 g/cm3. What volume (in cm3)would be occupied by a 21.8 g sample of osmium?
(Multiple Choice)
4.9/5  (32)
(32)
The correct result (indicating the proper number of significant figures)of the following problem is ________. 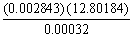
(Multiple Choice)
4.8/5  (45)
(45)
The correct answer (reported to the proper number of significant figures)to the following is ________. 6.3 × 3.25 = ________
(Multiple Choice)
4.8/5  (37)
(37)
Which one of the following has the element name and symbol correctly matched?
(Multiple Choice)
4.8/5  (33)
(33)
Osmium has a density of 22.6 g/cm3. The mass of a block of osmium that measures 1.01 cm × 0.233 cm × 0.648 cm is ________ g.
(Multiple Choice)
4.8/5  (34)
(34)
The density of a gold nugget is 19.3 g/cm3. If the volume of the gold nugget is 0.00369 L, the mass of the nugget is ________ g.
(Multiple Choice)
4.8/5  (31)
(31)
Showing 61 - 80 of 163
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)