Exam 11: Liquids and Intermolecular Forces
Exam 1: Introduction: Matter and Measurement163 Questions
Exam 2: Atoms, Molecules, and Ions250 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations178 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry180 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding147 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry114 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
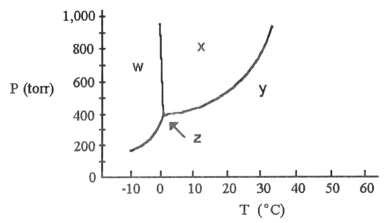 -According to the phase diagram shown above, the normal melting point of this substance is ________ °C.
-According to the phase diagram shown above, the normal melting point of this substance is ________ °C.
(Multiple Choice)
4.9/5  (45)
(45)
 -Based on the figure above, the boiling point of diethyl ether under an external pressure of 0.605 atm is ________ °C.
-Based on the figure above, the boiling point of diethyl ether under an external pressure of 0.605 atm is ________ °C.
(Multiple Choice)
4.8/5  (35)
(35)
Molecules with only single bonds do not generally exhibit liquid-crystalline properties because ________.
(Multiple Choice)
4.8/5  (37)
(37)
Which one of the following should have the lowest boiling point?
(Multiple Choice)
4.8/5  (43)
(43)
The critical temperature and pressure of CS2 are 279 °C and 78 atm, respectively. At temperatures above 279 °C and pressures above 78 atm, CS2 can only occur as a ________.
(Multiple Choice)
4.8/5  (31)
(31)
 -The phase diagram of a substance is shown above. The area labeled ________ indicates the solid phase for the substance.
-The phase diagram of a substance is shown above. The area labeled ________ indicates the solid phase for the substance.
(Multiple Choice)
4.8/5  (51)
(51)
With what compound will NH3 experience only dispersion intermolecular forces?
(Multiple Choice)
4.8/5  (36)
(36)
Heat of sublimation can be approximated by adding together ________ and ________.
(Multiple Choice)
4.9/5  (42)
(42)
Of the following substances, ________ has the highest boiling point.
(Multiple Choice)
4.7/5  (41)
(41)
Ethanol melts at -114 °C and boils at 78 °C at a constant pressure of 1 atm. What state of matter must a sample of ethanol be in at 0°C and 1 atm?
(Multiple Choice)
4.9/5  (26)
(26)
Large intermolecular forces in a substance are manifested by ________.
(Multiple Choice)
4.8/5  (39)
(39)
Which one of the following exhibits dipole-dipole attraction between molecules?
(Multiple Choice)
4.9/5  (40)
(40)
Of the following substances, only ________ has London dispersion forces as its only intermolecular force.
(Multiple Choice)
4.7/5  (44)
(44)
 -The phase diagram of a substance is shown above. The area labeled ________ indicates the liquid phase for the substance.
-The phase diagram of a substance is shown above. The area labeled ________ indicates the liquid phase for the substance.
(Multiple Choice)
4.8/5  (42)
(42)
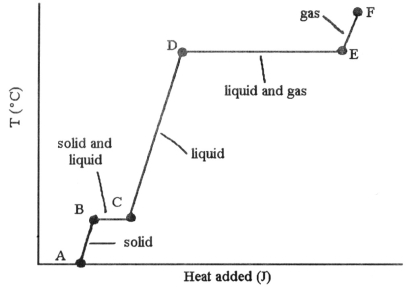 -The heating curve shown was generated by measuring the heat flow and temperature of a solid as it was heated. The heat flow into the sample in the segment ________ will yield the value of the ΔHvap of this substance.
-The heating curve shown was generated by measuring the heat flow and temperature of a solid as it was heated. The heat flow into the sample in the segment ________ will yield the value of the ΔHvap of this substance.
(Multiple Choice)
4.8/5  (35)
(35)
Of the following substances, only ________ has London dispersion forces as the only intermolecular force.
(Multiple Choice)
4.9/5  (31)
(31)
Showing 21 - 40 of 124
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)