Exam 11: Liquids and Intermolecular Forces
Exam 1: Introduction: Matter and Measurement163 Questions
Exam 2: Atoms, Molecules, and Ions250 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations178 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry180 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding147 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry114 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
In which of the following molecules is hydrogen bonding likely to be the most significant component of the total intermolecular forces?
(Multiple Choice)
4.8/5  (40)
(40)
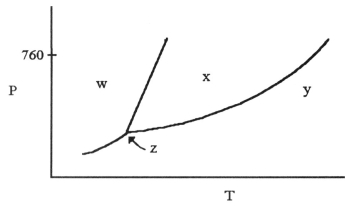 -The phase diagram of a substance is given above. The region that corresponds to the solid phase is ________.
-The phase diagram of a substance is given above. The region that corresponds to the solid phase is ________.
(Multiple Choice)
4.9/5  (37)
(37)
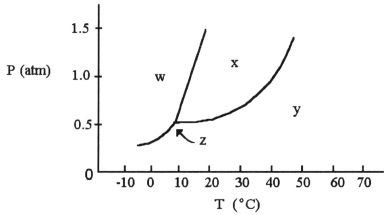 -The normal boiling point of the substance with the phase diagram shown above is ________ °C.
-The normal boiling point of the substance with the phase diagram shown above is ________ °C.
(Multiple Choice)
4.9/5  (36)
(36)
What types of intermolecular forces exist between NH3 and HF?
(Multiple Choice)
4.9/5  (49)
(49)
The intermolecular force(s)responsible for the fact that CH4 has the lowest boiling point in the set CH4, SiH4, GeH4, SnH4 is/are ________.
(Multiple Choice)
4.8/5  (33)
(33)
Of the following substances, ________ has the highest boiling point.
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following molecules has hydrogen bonding as its only intermolecular force?
(Multiple Choice)
4.9/5  (31)
(31)
For a given substance that exhibits liquid-crystalline properties, the transition from solid to liquid-crystal state occurs ________.
(Multiple Choice)
4.9/5  (32)
(32)
Boron triiodide (BI3)melts at 49.9 °C and boils at 209.5 °C at a constant pressure of 1 atm. What state of matter must a sample of boron triiodide be in at 100°C and 1 atm?
(Multiple Choice)
4.9/5  (31)
(31)
What intermolecular force is responsible for the fact that ice is less dense than liquid water?
(Multiple Choice)
4.8/5  (37)
(37)
As a gaseous element condenses, the atoms become ________ and they have ________ attraction for one another.
(Multiple Choice)
5.0/5  (40)
(40)
A ________ liquid crystal has the least order and is the most liquid-like.
(Multiple Choice)
4.9/5  (39)
(39)
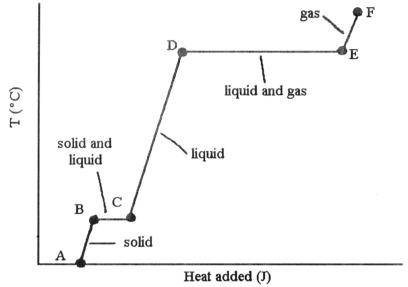 -The phase changes B → C and D → E are not associated with temperature increases because the heat energy is used up to ________.
-The phase changes B → C and D → E are not associated with temperature increases because the heat energy is used up to ________.
(Multiple Choice)
4.8/5  (33)
(33)
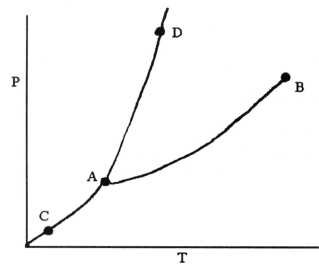 -On the phase diagram shown above, the coordinates of point ________ correspond to the critical temperature and pressure.
-On the phase diagram shown above, the coordinates of point ________ correspond to the critical temperature and pressure.
(Multiple Choice)
4.8/5  (39)
(39)
How high a liquid will rise up a narrow tube as a result of capillary action depends on ________.
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following characteristics would prevent liquid crystal behavior?
(Multiple Choice)
4.9/5  (40)
(40)
What type(s)of intermolecular forces exist between Br2 and CCl4?
(Multiple Choice)
4.9/5  (43)
(43)
In liquids, the attractive intermolecular forces are ________.
(Multiple Choice)
4.8/5  (37)
(37)
Showing 41 - 60 of 124
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)