Exam 23: Transition Metals and Coordination Chemistry
Exam 1: Introduction: Matter and Measurement151 Questions
Exam 2: Atoms, Molecules, and Ions230 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations170 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry177 Questions
Exam 5: Thermochemistry148 Questions
Exam 6: Electronic Structure of Atoms180 Questions
Exam 7: Periodic Properties of the Elements171 Questions
Exam 8: Basic Concepts of Chemical Bonding141 Questions
Exam 9: Molecular Geometry and Bonding Theories177 Questions
Exam 10: Gases172 Questions
Exam 11: Liquids and Intermolecular Forces119 Questions
Exam 12: Solids and Modern Materials78 Questions
Exam 13: Properties of Solutions151 Questions
Exam 14: Chemical Kinetics130 Questions
Exam 15: Chemical Equilibrium92 Questions
Exam 16: Acid-Base Equilibria134 Questions
Exam 17: Additional Aspects of Aqueous Equilibria111 Questions
Exam 18: Chemistry of the Environment121 Questions
Exam 19: Chemical Thermodynamics120 Questions
Exam 20: Electrochemistry110 Questions
Exam 21: Nuclear Chemistry158 Questions
Exam 22: Chemistry of the Nonmetals192 Questions
Exam 23: Transition Metals and Coordination Chemistry147 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry124 Questions
Select questions type
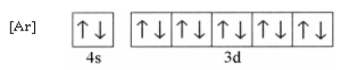
Draw a diagram of the short-hand ground state electron configuration of zinc.
Free
(Essay)
4.7/5  (27)
(27)
Correct Answer:
Which of the following statements is (are)false?
Free
(Multiple Choice)
4.8/5  (39)
(39)
Correct Answer:
D
How does an elevated body temperature deprive some bacteria in the body of iron?
Free
(Essay)
4.8/5  (41)
(41)
Correct Answer:
In some bacteria, siderophore production decreases as temperature increases.
What is the oxidation number of chromium in [Cr(NH3)4Cl2]Cl?
(Multiple Choice)
5.0/5  (49)
(49)
Triphenylphosphine is often given the abbreviated formula PPh3. The correct name for Rh(PPh3)3Cl is __________.
(Multiple Choice)
4.7/5  (36)
(36)
The chelate effect is best attributed to considerations of which type?
(Multiple Choice)
4.9/5  (41)
(41)
Metals with __________ electron configurations characteristically form diamagnetic, square planar complexes.
(Multiple Choice)
4.7/5  (36)
(36)
How many ligands are there in the coordination sphere of [Co(en)2Cl2]+?
(Multiple Choice)
4.8/5  (33)
(33)
How can high-spin and low-spin transition metal complexes be distinguished from each other?
(Essay)
4.7/5  (31)
(31)
If chloride is a ligand to a transition metal it will not be precipitated by silver nitrate.
(True/False)
4.9/5  (42)
(42)
Which one of the following species is a potential polydentate ligand (chelating agent)?
(Multiple Choice)
4.9/5  (40)
(40)
During the formation of a coordination compound, ligands act as __________.
(Multiple Choice)
4.8/5  (45)
(45)
In the leaves of plants, visible light is absorbed by a compound known as __________, and is aided by a __________ ion bonded to a porphyrin ring.
(Short Answer)
4.8/5  (42)
(42)
The coordination number of cobalt in CoCl3 ∙ 6NH3 is __________.
(Multiple Choice)
4.8/5  (42)
(42)
A geometrical isomer with like groups located on opposite sides of the metal atom is denoted with the prefix __________.
(Multiple Choice)
4.8/5  (34)
(34)
Werner's theory of primary and secondary valences for transition metal complexes has given us the concepts of __________ and __________.
(Short Answer)
4.8/5  (26)
(26)
__________ arises when the unpaired electrons of the atoms or ions in a solid are influenced by the orientations of the electrons of their neighbors.
(Short Answer)
4.8/5  (31)
(31)
Showing 1 - 20 of 147
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)