Exam 1: Introduction: Matter and Measurement
Exam 1: Introduction: Matter and Measurement151 Questions
Exam 2: Atoms, Molecules, and Ions230 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations170 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry177 Questions
Exam 5: Thermochemistry148 Questions
Exam 6: Electronic Structure of Atoms180 Questions
Exam 7: Periodic Properties of the Elements171 Questions
Exam 8: Basic Concepts of Chemical Bonding141 Questions
Exam 9: Molecular Geometry and Bonding Theories177 Questions
Exam 10: Gases172 Questions
Exam 11: Liquids and Intermolecular Forces119 Questions
Exam 12: Solids and Modern Materials78 Questions
Exam 13: Properties of Solutions151 Questions
Exam 14: Chemical Kinetics130 Questions
Exam 15: Chemical Equilibrium92 Questions
Exam 16: Acid-Base Equilibria134 Questions
Exam 17: Additional Aspects of Aqueous Equilibria111 Questions
Exam 18: Chemistry of the Environment121 Questions
Exam 19: Chemical Thermodynamics120 Questions
Exam 20: Electrochemistry110 Questions
Exam 21: Nuclear Chemistry158 Questions
Exam 22: Chemistry of the Nonmetals192 Questions
Exam 23: Transition Metals and Coordination Chemistry147 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry124 Questions
Select questions type
Cu is the symbol for the element __________.
Free
(Short Answer)
4.7/5  (39)
(39)
Correct Answer:
Copper
What decimal power does the abbreviation pico represent?
Free
(Multiple Choice)
4.9/5  (30)
(30)
Correct Answer:
D
The density of mercury is 13.6 g/cm3. The density of mercury is __________ kg/m3.
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
B
Osmium has a density of 22.6 g/cm3. What volume (in cm3)would be occupied by a 21.8 g sample of osmium?
(Multiple Choice)
4.9/5  (35)
(35)
The unit of force in the English measurement system is 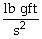 . The SI unit of force is the Newton, which is __________ in base SI units.
. The SI unit of force is the Newton, which is __________ in base SI units.
(Multiple Choice)
4.7/5  (34)
(34)
A certain liquid has a density of 2.67 g/cm3. 1340 g of this liquid would occupy a volume of __________ L.
(Multiple Choice)
4.8/5  (30)
(30)
If an object, beginning at rest, is moving at a speed of 700 m/s after 2.75 min, its rate of acceleration (in m/s2)is __________. (Assume that the rate of acceleration is constant.)
(Multiple Choice)
4.8/5  (28)
(28)
The density of air under ordinary conditions at 25 °C is 1.19 g/L. How many kilograms of air are in a room that measures 11.0 ft x 11.0 ft and has an 10.0 ft ceiling? 1 in. = 2.54 cm (exactly); 1 L = 103 cm3
(Multiple Choice)
4.9/5  (48)
(48)
The initial or tentative explanation of an observation is called a(n)__________.
(Multiple Choice)
4.9/5  (33)
(33)
How many significant figures should be retained in the result of the following calculation? 12.00000 × 0.9893 + 13.00335 × 0.0107
(Multiple Choice)
4.8/5  (33)
(33)
Temperature is a physical property that determines the direction of heat flow.
(True/False)
4.8/5  (29)
(29)
In the following list, only __________ is not an example of a chemical reaction.
(Multiple Choice)
4.7/5  (36)
(36)
Gold has a density of 0.01932 kg/cm3. What volume (in cm3)would be occupied by a 33.3 g sample of gold?
(Multiple Choice)
4.7/5  (33)
(33)
The correct answer (reported to the proper number of significant figures)to the following is __________.
(1815-1806)× (9.11 × 7.92)= __________
(Short Answer)
4.9/5  (36)
(36)
In which one of the following numbers are none of the zeros significant?
(Multiple Choice)
4.9/5  (37)
(37)
What is the volume (in cm3)of a 63.4 g piece of metal with a density of 12.86 g/cm3?
(Multiple Choice)
4.8/5  (33)
(33)
Showing 1 - 20 of 151
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)