Exam 6: Liquids and Solids
Exam 1: The Quantum World99 Questions
Exam 2: Quantum Mechanics in Action: Atoms100 Questions
Exam 3: Chemical Bonds84 Questions
Exam 4: Molecular Shape and Structure95 Questions
Exam 5: The Properties of Gases94 Questions
Exam 6: Liquids and Solids94 Questions
Exam 7: Inorganic Materials99 Questions
Exam 8: Thermodynamics: the First Law94 Questions
Exam 9: Thermodynamics: the Second and Third Laws93 Questions
Exam 10: Physical Equilibria94 Questions
Exam 11: Chemical Equilibria93 Questions
Exam 12: Acids and Bases94 Questions
Exam 13: Aqueous Equilibria94 Questions
Exam 14: Electrochemistry94 Questions
Exam 15: Chemical Kinetics88 Questions
Exam 16: The Elements: the Main Group Elements186 Questions
Exam 17: The Elements: the D Block93 Questions
Exam 18: Nuclear Chemistry95 Questions
Exam 19: Organic Chemistry I93 Questions
Exam 20: Organic Chemistry II94 Questions
Select questions type
In the rock-salt structure (NaCl),the cations occupy all the octahedral holes.True or false?
(True/False)
4.8/5  (35)
(35)
What are the coordination numbers of Ti4+ and O2,respectively,in rutile? The unit cell is shown below. 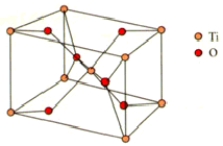
(Multiple Choice)
4.7/5  (46)
(46)
If a cubic unit cell of an ionic compound has A cations at the corners and the face centers and X anions in the centers of the edges,what is the empirical formula of the compound?
(Multiple Choice)
4.8/5  (43)
(43)
How many tetrahedral and octahedral holes per atom are there in a cubic close-packed structure,respectively?
(Multiple Choice)
4.7/5  (28)
(28)
The atomic radius of aluminum is 143 pm.Estimate its density,given that the metal has a close-packed structure.
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following can form intermolecular hydrogen bonds?
(Multiple Choice)
4.8/5  (41)
(41)
If the interaction between two species is proportional to 1/r2,which of the following is likely involved?
(Multiple Choice)
4.9/5  (41)
(41)
Order the following cubic unit cells from highest percentage of empty space to lowest: body-centered,primitive,face-centered.
(Multiple Choice)
4.7/5  (38)
(38)
Match each statement with the intermolecular force mainly responsible for the phenomenon.
1.Helium can be liquefied. (a)dipole-dipole
2.Sodium salts are commonly hydrated. (b)hydrogen bonding
3.The boiling point of cis-dichloroethene
is higher than that of trans-dichloroethene. (c)London dispersion
4.NH3 has a higher boiling point than PH3. (d)ion-dipole
(Short Answer)
4.9/5  (40)
(40)
Superconductivity is the loss of all electrical resistance when a substance is cooled below a certain characteristic transition temperature.True or false?
(True/False)
4.9/5  (37)
(37)
Which of the following can form intermolecular hydrogen bonds?
(Multiple Choice)
4.8/5  (26)
(26)
Sodium has a body-centered cubic unit cell.If the atomic radius of sodium is 190 pm,what is the density of sodium?
(Short Answer)
4.9/5  (45)
(45)
Glycerol,C3H8O3,has a higher viscosity than propanol,C3H8O.True or false?
(True/False)
4.9/5  (39)
(39)
Predict which of the following has the lowest boiling point.
(Multiple Choice)
4.7/5  (36)
(36)
What are all the intermolecular forces that are responsible for the existence of the molecular solid oxalic acid,H2C2O4?
(Multiple Choice)
4.8/5  (38)
(38)
How many calcium,titanium,and oxygen ions are there in the perovskite unit cell shown below? 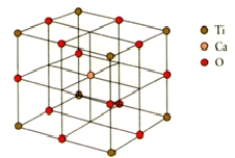
(Multiple Choice)
4.9/5  (40)
(40)
Showing 21 - 40 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)