Exam 1: Matter, measurements, and Calculations
Exam 1: Matter, measurements, and Calculations92 Questions
Exam 2: Atoms and Molecules89 Questions
Exam 3: Electronic Structure and the Periodic Law86 Questions
Exam 4: Forces Between Particles89 Questions
Exam 5: Chemical Reactions89 Questions
Exam 6: The States of Matter89 Questions
Exam 7: Solutions and Colloids88 Questions
Exam 8: Reaction Rates and Equilibrium87 Questions
Exam 9: Acids,bases,and Salts86 Questions
Exam 10: Radioactivity and Nuclear Processes86 Questions
Exam 11: Organic Compounds: Alkanes87 Questions
Exam 12: Unsaturated Hydrocarbons88 Questions
Exam 13: Alcohols, phenols, and Ethers87 Questions
Exam 14: Aldehydes and Ketones86 Questions
Exam 15: Carboxylic Acids and Esters86 Questions
Exam 16: Amines and Amides85 Questions
Exam 17: Carbohydrates88 Questions
Exam 18: Lipids91 Questions
Exam 19: Proteins89 Questions
Exam 20: Enzymes88 Questions
Exam 21: Nucleic Acids and Protein Synthesis90 Questions
Exam 22: Nutrition and Energy for Life89 Questions
Exam 23: Carbohydrate Metabolism90 Questions
Exam 24: Lipid and Amino Acid Metabolism94 Questions
Exam 25: Body Fluids86 Questions
Select questions type
Which of the following substances are composed of heteroatomic molecules?
(Multiple Choice)
4.9/5  (35)
(35)
Body density can be used to determine the amount of fat carried by an individual because the density of muscle is greater than that of fat.
(True/False)
4.9/5  (35)
(35)
If 13% of a class cheats on an exam and there are 93 students in the class,how many students should you recommend be expelled (to the nearest whole student)?
(Multiple Choice)
4.7/5  (44)
(44)
Molarity (M)is calculated as: 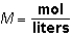 .M would be considered a derived unit.
.M would be considered a derived unit.
(True/False)
5.0/5  (42)
(42)
Based on data obtained in an experiment,to determine the density of a metal,the following calculation is carried out.Express the answer to the correct number of significant figures. 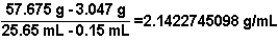
(Multiple Choice)
4.9/5  (42)
(42)
A patient with a body temperature of 300 K would be considered as suffering from hypothermia.
(True/False)
4.9/5  (41)
(41)
How is the weight of an object influenced when the gravitational force on the object is increased?
(Multiple Choice)
4.8/5  (38)
(38)
Which number has the greatest number of significant digits?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following set-ups will allow you to calculate the cost of fruit in dollars per gram,if the price is given as 0.79 dollars per pound?
(Multiple Choice)
4.8/5  (38)
(38)
The number twelve,representing a dozen,has two significant figures.
(True/False)
4.9/5  (49)
(49)
Do the following calculation and express the answer using the correct number of significant figures.
______ = (1.21 *10-3 + 1.3 *10-3)* 6.453 *102
(Multiple Choice)
4.8/5  (38)
(38)
If a student completes 5 problems out of a total of 8 on a pop quiz,what percentage of the quiz was completed?
(Multiple Choice)
4.7/5  (33)
(33)
If urine has a density of 1.08 g/mL,what would be the mass of a 125 mL urine sample?
(Multiple Choice)
4.9/5  (32)
(32)
On a cold winter day the weather report gives the temperature as -5.0° F.What would this temperature be if reported on the Kevin scale?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following consists of a single chemical species?
(Multiple Choice)
4.8/5  (27)
(27)
Showing 61 - 80 of 92
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)