Exam 2: Atoms and Molecules
Exam 1: Matter, measurements, and Calculations92 Questions
Exam 2: Atoms and Molecules89 Questions
Exam 3: Electronic Structure and the Periodic Law86 Questions
Exam 4: Forces Between Particles89 Questions
Exam 5: Chemical Reactions89 Questions
Exam 6: The States of Matter89 Questions
Exam 7: Solutions and Colloids88 Questions
Exam 8: Reaction Rates and Equilibrium87 Questions
Exam 9: Acids,bases,and Salts86 Questions
Exam 10: Radioactivity and Nuclear Processes86 Questions
Exam 11: Organic Compounds: Alkanes87 Questions
Exam 12: Unsaturated Hydrocarbons88 Questions
Exam 13: Alcohols, phenols, and Ethers87 Questions
Exam 14: Aldehydes and Ketones86 Questions
Exam 15: Carboxylic Acids and Esters86 Questions
Exam 16: Amines and Amides85 Questions
Exam 17: Carbohydrates88 Questions
Exam 18: Lipids91 Questions
Exam 19: Proteins89 Questions
Exam 20: Enzymes88 Questions
Exam 21: Nucleic Acids and Protein Synthesis90 Questions
Exam 22: Nutrition and Energy for Life89 Questions
Exam 23: Carbohydrate Metabolism90 Questions
Exam 24: Lipid and Amino Acid Metabolism94 Questions
Exam 25: Body Fluids86 Questions
Select questions type
One mole of silver would contain the same number of atoms as a mole of gold.
(True/False)
4.9/5  (29)
(29)
The symbols for all of the elements are derived from the Latin names.
(True/False)
5.0/5  (38)
(38)
How many neutrons are in an atom that has a mass number of 75 and contains 35 protons?
(Multiple Choice)
4.8/5  (38)
(38)
An MRI instrument cause the hydrogens in your body to line up because they are exposed to a very strong magnetic field.
(True/False)
4.9/5  (41)
(41)
If you have 3.011 *1023 atoms of carbon,what would you expect its mass to be?
(Multiple Choice)
4.8/5  (36)
(36)
The mass of mercury (Hg),a liquid at room temperature,is 200.6 amu/mol.A 200.6 gram sample of mercury is heated until it boils.What is the mass of one mole of mercury vapor (gas)?
(Multiple Choice)
4.8/5  (38)
(38)
An isotope of gallium consisting of 31 protons and 37 neutrons can be represented using the symbol shown below. 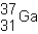
(True/False)
4.9/5  (42)
(42)
Electrons do not make an important contribution to the mass of an atom.
(True/False)
4.7/5  (30)
(30)
A mole of copper contains the same number of atoms as a mole of zinc.
(True/False)
4.8/5  (34)
(34)
How many moles of N2O contain the same number of nitrogen atoms as 4.60 g of NO2?
(Multiple Choice)
4.9/5  (38)
(38)
How many electrons are in a neutral atom of carbon-13 (13C)?
(Multiple Choice)
4.9/5  (32)
(32)
Atoms that have the same atomic number but differ by mass number are called?
(Multiple Choice)
4.8/5  (43)
(43)
Showing 41 - 60 of 89
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)