Exam 7: Solutions and Colloids
Exam 1: Matter, measurements, and Calculations92 Questions
Exam 2: Atoms and Molecules89 Questions
Exam 3: Electronic Structure and the Periodic Law86 Questions
Exam 4: Forces Between Particles89 Questions
Exam 5: Chemical Reactions89 Questions
Exam 6: The States of Matter89 Questions
Exam 7: Solutions and Colloids88 Questions
Exam 8: Reaction Rates and Equilibrium87 Questions
Exam 9: Acids,bases,and Salts86 Questions
Exam 10: Radioactivity and Nuclear Processes86 Questions
Exam 11: Organic Compounds: Alkanes87 Questions
Exam 12: Unsaturated Hydrocarbons88 Questions
Exam 13: Alcohols, phenols, and Ethers87 Questions
Exam 14: Aldehydes and Ketones86 Questions
Exam 15: Carboxylic Acids and Esters86 Questions
Exam 16: Amines and Amides85 Questions
Exam 17: Carbohydrates88 Questions
Exam 18: Lipids91 Questions
Exam 19: Proteins89 Questions
Exam 20: Enzymes88 Questions
Exam 21: Nucleic Acids and Protein Synthesis90 Questions
Exam 22: Nutrition and Energy for Life89 Questions
Exam 23: Carbohydrate Metabolism90 Questions
Exam 24: Lipid and Amino Acid Metabolism94 Questions
Exam 25: Body Fluids86 Questions
Select questions type
When will carbon dioxide in a carbonated soft drink dissolve best?
(Multiple Choice)
4.7/5  (27)
(27)
Which of the following would be considered a strong electrolyte?
(Multiple Choice)
4.7/5  (39)
(39)
When solid NaOH is dissolved in water,the solution becomes hot.The solution process is
(Multiple Choice)
4.9/5  (31)
(31)
When a patient's blood electrolyte levels are evaluated,sodium,chloride and bicarbonate ions are commonly measured and the difference in the total positive and negative charges calculated.This difference is called the
(Multiple Choice)
4.7/5  (30)
(30)
What volume of a 10.00% (w/v)solution of sugar is needed to provide 2.00 g of sugar?
(Multiple Choice)
4.8/5  (32)
(32)
What is the osmotic pressure of a 0.050 M solution of AlCl3 in water that is at 0.00 C? Consider AlCl3 to be a strong electrolyte.
(Multiple Choice)
4.9/5  (38)
(38)
You want to remove as much CO2 gas as possible from a water solution.Which of the following treatments would be most effective?
(Multiple Choice)
4.8/5  (42)
(42)
Light scattering is an effective way to distinguish between true solutions and colloidal dispersions.
(True/False)
4.7/5  (37)
(37)
When preparing a 2.00 M solution of NaCl in water,a volumetric flask could be used to measure the volume of the solution.
(True/False)
5.0/5  (33)
(33)
Which of the following pairs correctly represent similar functions for a solution component and a colloid component?
(Multiple Choice)
4.8/5  (32)
(32)
Express the following concentration of solution in terms of molarity: 3.00 L of solution contains 1.75 mol of solute.
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following statements relating to a solution is not correct?
(Multiple Choice)
4.8/5  (40)
(40)
When sodium acetate dissolves in water,the following reaction occurs. 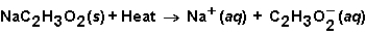 This solution could be used
This solution could be used
(Multiple Choice)
4.9/5  (41)
(41)
Rock candy (large table sugar crystals)can be produced by allowing a hot,saturated solution of sugar in water to cool off.
(True/False)
4.8/5  (33)
(33)
A solution is produced in which water is the solvent and there are four solutes.Which of the solutes can dissolve better if the solution is heated?
(Multiple Choice)
4.8/5  (31)
(31)
Iodine,I2,is very slightly soluble in water,a polar solvent,but quite soluble in toluene,a nonpolar solvent.What can be inferred about the nature of the I2 molecule?
(Multiple Choice)
4.9/5  (41)
(41)
Showing 21 - 40 of 88
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)