Exam 10: Radioactivity and Nuclear Processes
Exam 1: Matter, measurements, and Calculations92 Questions
Exam 2: Atoms and Molecules89 Questions
Exam 3: Electronic Structure and the Periodic Law86 Questions
Exam 4: Forces Between Particles89 Questions
Exam 5: Chemical Reactions89 Questions
Exam 6: The States of Matter89 Questions
Exam 7: Solutions and Colloids88 Questions
Exam 8: Reaction Rates and Equilibrium87 Questions
Exam 9: Acids,bases,and Salts86 Questions
Exam 10: Radioactivity and Nuclear Processes86 Questions
Exam 11: Organic Compounds: Alkanes87 Questions
Exam 12: Unsaturated Hydrocarbons88 Questions
Exam 13: Alcohols, phenols, and Ethers87 Questions
Exam 14: Aldehydes and Ketones86 Questions
Exam 15: Carboxylic Acids and Esters86 Questions
Exam 16: Amines and Amides85 Questions
Exam 17: Carbohydrates88 Questions
Exam 18: Lipids91 Questions
Exam 19: Proteins89 Questions
Exam 20: Enzymes88 Questions
Exam 21: Nucleic Acids and Protein Synthesis90 Questions
Exam 22: Nutrition and Energy for Life89 Questions
Exam 23: Carbohydrate Metabolism90 Questions
Exam 24: Lipid and Amino Acid Metabolism94 Questions
Exam 25: Body Fluids86 Questions
Select questions type
You inject a 160 lb male patient with 1.0 ml of a saline solution containing a radioactive form of sodium.It has an activity of 5.0 * 104 dpm.After allowing sufficient time for the solution to mix,you remove a 1.0 ml sample of blood and measure its radioactivity.You discover it to have an activity of 11 dpm.What is the patient's blood volume? (Hint: this is similar to a M1V1 = M2V2 type problem. )
(Multiple Choice)
4.8/5  (34)
(34)
The gray is one of several units used in association with the biological impact of radiation exposure.
(True/False)
4.7/5  (38)
(38)
The reasons why I-131 is not suitable for use as a tracer are the same reasons why I-131 is an appropriate choice to treat thyroid cancer.
(True/False)
4.7/5  (37)
(37)
A good radioisotope tracer for medical use should not have the following characteristic.
(Multiple Choice)
4.8/5  (39)
(39)
Most of the synthetic (man-made)elements are stable and have been produced in large amounts.
(True/False)
4.9/5  (39)
(39)
Which of the following would be associated with medical testing?
(Multiple Choice)
4.7/5  (28)
(28)
In nuclear reactions,the principle change occurs in the ______.
(Multiple Choice)
4.9/5  (39)
(39)
The following equation describes the amount of energy released in nuclear reactions.
E = mc2
(True/False)
4.8/5  (47)
(47)
A rem is a biological radiation measurement which is independent of the type of radiation.
(True/False)
4.8/5  (36)
(36)
Manganese can undergo several types of radioactive emission.In which type of decay does the following reaction occur? 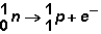
(Multiple Choice)
4.8/5  (33)
(33)
Radioisotopes which emit alpha rays make the best diagnostic tracers.
(True/False)
4.8/5  (40)
(40)
Which form of radiation would not be deflected by a magnetic field?
(Multiple Choice)
4.8/5  (31)
(31)
A nuclear reaction in which beta particles are emitted yields an atom that weighs 2 less than the starting element.
(True/False)
4.8/5  (30)
(30)
Carbon-14 dating is a useful tool for determining the age of the artifacts of ancient civilizations.However,when future archeologists study ruins of our civilization,they obtain confusing results -- with some artifacts dating MUCH older than others.Which of the following would be an explanation for these results?
(Multiple Choice)
4.8/5  (38)
(38)
Which is the explanation for the difference between the two isotopes of carbon,C-12 and C-14?
(Multiple Choice)
4.8/5  (39)
(39)
Showing 41 - 60 of 86
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)