Exam 19: Organic Compounds
Exam 1: Patterns of Motion and Equilibrium94 Questions
Exam 2: Newtons Laws of Motion109 Questions
Exam 3: Momentum and Energy128 Questions
Exam 4: Gravity, Projectiles, and Satellites114 Questions
Exam 5: Fluid Mechanics120 Questions
Exam 6: Thermal Energy and Thermodynamics100 Questions
Exam 7: Heat Transfer and Change of Phase115 Questions
Exam 8: Static and Current Electricity144 Questions
Exam 9: Magnetism and Electromagnetic Induction105 Questions
Exam 10: Waves and Sound120 Questions
Exam 11: Light146 Questions
Exam 12: Atoms and the Periodic Table128 Questions
Exam 13: The Atomic Nucleus and Radioactivity124 Questions
Exam 14: Elements of Chemistry49 Questions
Exam 15: How Atoms Bond and Molecules Attract150 Questions
Exam 16: Mixtures141 Questions
Exam 17: How Chemicals React118 Questions
Exam 18: Two Classes of Chemical Reactions182 Questions
Exam 19: Organic Compounds98 Questions
Exam 20: Rocks and Minerals170 Questions
Exam 21: Plate Tectonics and Earths Interior175 Questions
Exam 22: Shaping Earths Surface175 Questions
Exam 23: Geologic Timereading the Rock Record145 Questions
Exam 24: The Oceans, Atmosphere, and Climatic Effects172 Questions
Exam 25: Driving Forces of Weather145 Questions
Exam 26: The Solar System87 Questions
Exam 27: Stars and Galaxies84 Questions
Exam 28: The Structure of Space and Time55 Questions
Exam 29: Prologue: the Nature of Science22 Questions
Select questions type
Why might a high-formula-mass alcohol be insoluble in water?
Free
(Multiple Choice)
4.7/5  (35)
(35)
Correct Answer:
B
In a fractionating tower, the crude oil vapors pass from a pipe still into the column. Tar and lubricating stock are the first components to be pulled off at the bottom. Nearer the top kerosene is pulled off followed by gasoline and finally natural gas at the very top. From this information, which has a higher boiling point, gasoline or kerosine?
Free
(Multiple Choice)
4.9/5  (29)
(29)
Correct Answer:
B
The temperatures in a fractionating tower at an oil refinery are important, but so are the pressures. Where might the pressure in a fractional distillation tower be greatest, at the bottom or at the top? Defend your answer.
Free
(Multiple Choice)
4.7/5  (27)
(27)
Correct Answer:
A
Where would you expect to isolate molecules in a fractionating tower that have a large number of interatomic forces?
(Multiple Choice)
4.9/5  (27)
(27)
In water, does the molecule lysergic acid diethylamide act as an acid, a base, neither or both? 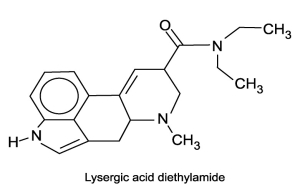
(Multiple Choice)
4.9/5  (41)
(41)
Heteroatoms make a difference in the physical and chemical properties of an organic molecule because
(Multiple Choice)
4.7/5  (33)
(33)
Which of the monomers below would be used to make the following polymer? 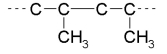
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following is the hallmark of a saturated hydrocarbon?
(Multiple Choice)
4.7/5  (31)
(31)
What type of polymer would be best to use in the manufacture of stain-resistant carpets?
(Multiple Choice)
4.8/5  (35)
(35)
Which of the above molecules would most likely have a strong, distinct smell?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following stick structures could describe pentane (C5H12)? 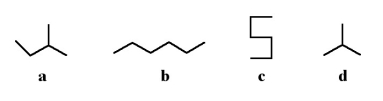
(Multiple Choice)
4.9/5  (40)
(40)
What is the main difference between High Density PolyEtyhlene (HDPE)and Low Density PolyEthylene(LDPE)?
(Multiple Choice)
4.8/5  (35)
(35)
Which two of these four structures are of the same structural isomer? 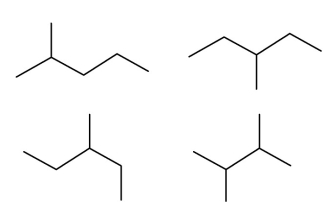
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following two stick structures are structural isomers? 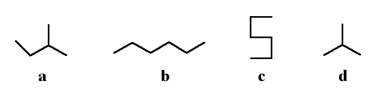
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following molecules could be a different conformation of the underlined molecule below? 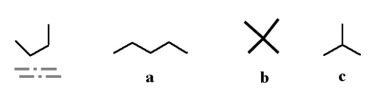
(Multiple Choice)
4.9/5  (41)
(41)
Showing 1 - 20 of 98
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)