Exam 13: The Atomic Nucleus and Radioactivity
Exam 1: Patterns of Motion and Equilibrium94 Questions
Exam 2: Newtons Laws of Motion109 Questions
Exam 3: Momentum and Energy128 Questions
Exam 4: Gravity, Projectiles, and Satellites114 Questions
Exam 5: Fluid Mechanics120 Questions
Exam 6: Thermal Energy and Thermodynamics100 Questions
Exam 7: Heat Transfer and Change of Phase115 Questions
Exam 8: Static and Current Electricity144 Questions
Exam 9: Magnetism and Electromagnetic Induction105 Questions
Exam 10: Waves and Sound120 Questions
Exam 11: Light146 Questions
Exam 12: Atoms and the Periodic Table128 Questions
Exam 13: The Atomic Nucleus and Radioactivity124 Questions
Exam 14: Elements of Chemistry49 Questions
Exam 15: How Atoms Bond and Molecules Attract150 Questions
Exam 16: Mixtures141 Questions
Exam 17: How Chemicals React118 Questions
Exam 18: Two Classes of Chemical Reactions182 Questions
Exam 19: Organic Compounds98 Questions
Exam 20: Rocks and Minerals170 Questions
Exam 21: Plate Tectonics and Earths Interior175 Questions
Exam 22: Shaping Earths Surface175 Questions
Exam 23: Geologic Timereading the Rock Record145 Questions
Exam 24: The Oceans, Atmosphere, and Climatic Effects172 Questions
Exam 25: Driving Forces of Weather145 Questions
Exam 26: The Solar System87 Questions
Exam 27: Stars and Galaxies84 Questions
Exam 28: The Structure of Space and Time55 Questions
Exam 29: Prologue: the Nature of Science22 Questions
Select questions type
To predict the approximate energy release of either a fission or a fusion reaction, explain how a physicist uses a table of nuclear masses and the equation E = m 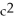 .
.
Free
(Multiple Choice)
4.9/5  (29)
(29)
Correct Answer:
B
In bombarding atomic nuclei with proton "bullets," the protons must be accelerated to high energies to make contact with the target nuclei
Free
(Multiple Choice)
4.9/5  (19)
(19)
Correct Answer:
D
Which of the beams is actually composed of particles?
Free
(Multiple Choice)
4.9/5  (28)
(28)
Correct Answer:
D
You are given three radioactive samples (α, β, and γ)and can only dispose of one. The other two you must keep, one in your hand, the other in your pocket. How would you minimize your exposure risk?
(Multiple Choice)
4.9/5  (31)
(31)
Why might the following nuclear reaction not be very good for energy production in a fission reactor?  Fe →
Fe →  Si +
Si +  Mg + 3 neutrons
Mg + 3 neutrons
(Multiple Choice)
4.9/5  (42)
(42)
How is it possible for an element to decay "forward in the periodic table"--that is, to decay to an element of higher atomic number?
(Multiple Choice)
4.8/5  (33)
(33)
Is it at all possible for a hydrogen nucleus to emit an alpha particle?
(Multiple Choice)
4.9/5  (29)
(29)
The half-life of carbon-14 is 5,730 years. A sample is found to have one-eighth the original amount of carbon-14 in it. How old is the sample?
(Multiple Choice)
4.9/5  (41)
(41)
Which shape is likely to need more material for a critical mass, a cube or a sphere? Why?
(Multiple Choice)
4.9/5  (37)
(37)
A certain radioactive element has a half-life of one hour. If you start with a 1-g sample of the element at noon, how much of this same element will be left at 3:00 PM?
(Multiple Choice)
4.8/5  (24)
(24)
The alpha particle has twice the electric charge of the beta particle but deflects less than the beta in a magnetic field because it
(Multiple Choice)
4.9/5  (29)
(29)
Why might the following nuclear reaction not be very good for energy production in a fission reactor? 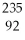 U →
U → 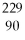 Th +
Th + 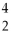 He + 3 neutrons
He + 3 neutrons
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following is a major advantage of nuclear-fission-based power plants?
(Multiple Choice)
4.6/5  (28)
(28)
Why is the combination of two protons and two neutrons stable, but two protons and one neutron is not?
(Multiple Choice)
4.9/5  (30)
(30)
The original reactor built in 1942 was just "barely" critical because the natural uranium that was used contained less than 1% of the fissionable isotope U-235 (half life 713 million years). What if, in 1942, the Earth had been 9 billion years old instead of 4.5 billion years old? Would this reactor have reached critical stage with natural uranium? Why?
(Multiple Choice)
4.8/5  (42)
(42)
A pair of protons in an atomic nucleus repel each other, but they are also attracted to each other. Why?
(Multiple Choice)
4.8/5  (32)
(32)
Why are smaller nuclei such as carbon-14 often radioactive? (carbon-12 is the most common and most stable form of carbon.)
(Multiple Choice)
4.9/5  (39)
(39)
Showing 1 - 20 of 124
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)