Exam 12: Atoms and the Periodic Table
Exam 1: Patterns of Motion and Equilibrium94 Questions
Exam 2: Newtons Laws of Motion109 Questions
Exam 3: Momentum and Energy128 Questions
Exam 4: Gravity, Projectiles, and Satellites114 Questions
Exam 5: Fluid Mechanics120 Questions
Exam 6: Thermal Energy and Thermodynamics100 Questions
Exam 7: Heat Transfer and Change of Phase115 Questions
Exam 8: Static and Current Electricity144 Questions
Exam 9: Magnetism and Electromagnetic Induction105 Questions
Exam 10: Waves and Sound120 Questions
Exam 11: Light146 Questions
Exam 12: Atoms and the Periodic Table128 Questions
Exam 13: The Atomic Nucleus and Radioactivity124 Questions
Exam 14: Elements of Chemistry49 Questions
Exam 15: How Atoms Bond and Molecules Attract150 Questions
Exam 16: Mixtures141 Questions
Exam 17: How Chemicals React118 Questions
Exam 18: Two Classes of Chemical Reactions182 Questions
Exam 19: Organic Compounds98 Questions
Exam 20: Rocks and Minerals170 Questions
Exam 21: Plate Tectonics and Earths Interior175 Questions
Exam 22: Shaping Earths Surface175 Questions
Exam 23: Geologic Timereading the Rock Record145 Questions
Exam 24: The Oceans, Atmosphere, and Climatic Effects172 Questions
Exam 25: Driving Forces of Weather145 Questions
Exam 26: The Solar System87 Questions
Exam 27: Stars and Galaxies84 Questions
Exam 28: The Structure of Space and Time55 Questions
Exam 29: Prologue: the Nature of Science22 Questions
Select questions type
The oldest known elements in the periodic table are the ones with
Free
(Multiple Choice)
4.7/5  (29)
(29)
Correct Answer:
C
Which of the following elements is a transition metal?
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
D
How do we account for the great variety of substances in the world?
Free
(Multiple Choice)
4.8/5  (28)
(28)
Correct Answer:
D
Some older cars vibrate loudly when driving at particular speeds. For example, at 65 mph the car may be most quiet, but at 60 mph the car rattles uncomfortably. How is this analogous to the quantized energy levels of an electron in an atom?
(Multiple Choice)
4.7/5  (35)
(35)
If an element has 15 protons and 16 neutrons and 15 electrons, what is the atomic mass of the element?
(Multiple Choice)
4.8/5  (37)
(37)
If an atom were the size of a baseball, its nucleus would be about the size of a(n)
(Multiple Choice)
4.8/5  (32)
(32)
Consider the various frequencies of the three photons emitted from the following three individual electron transitions in the figure below: n=3 to n=2; n=2 to n=1; n=3 to n=1. These transitions would produce three spectral lines in a spectroscope. If the energy spacing between the levels were equal, would this affect the number of spectral lines? 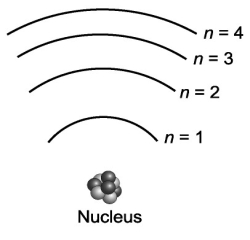
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following best describes a conceptual model of an atom?
(Multiple Choice)
4.9/5  (33)
(33)
How might you distinguish a sodium-vapor street lamp from a mercury-vapor street lamp?
(Multiple Choice)
4.8/5  (40)
(40)
Germanium, Ge (number 32), computer chips operate faster than silicon, Si (number 14), computer chips. So how might a gallium, Ga (number 31), chip compare with a germanium chip?
(Multiple Choice)
4.9/5  (30)
(30)
What is the main difference between a conceptual model and a physical model?
(Multiple Choice)
4.9/5  (37)
(37)
The following statement describes which subatomic particle best? It is electrically charged.
(Multiple Choice)
4.8/5  (28)
(28)
How many electrons are there in the third shell of sodium, Na (atomic number 11)?
(Multiple Choice)
4.8/5  (26)
(26)
Showing 1 - 20 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)