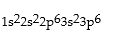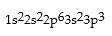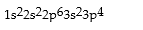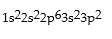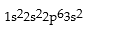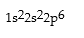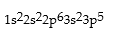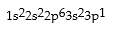Exam 4: The Modern Model of the Atom
Exam 1: What Is Chemistry51 Questions
Exam 2: The Numerical Side of Chemistry147 Questions
Exam 3: The Evolution of Atomic Theory92 Questions
Exam 4: The Modern Model of the Atom97 Questions
Exam 5: Chemical Bonding and Nomenclature245 Questions
Exam 6: The Shape of Molecules92 Questions
Exam 7: Intermolecular Forces and the Phases of Matter67 Questions
Exam 8: Chemical Reactions75 Questions
Exam 9: Stoichiometry and the Mole191 Questions
Exam 10: Electron Transfer in Chemical Reactions87 Questions
Exam 11: What If There Were No Intermolecular Forces the Ideal Gas80 Questions
Exam 12: Solutions92 Questions
Exam 13: When Reactants Turn Into Products79 Questions
Exam 14: Chemical Equilibrium94 Questions
Exam 15: Electrolytes, Acids, and Bases94 Questions
Exam 16: Nuclear Chemistry84 Questions
Exam 17: The Chemistry of Carbon71 Questions
Exam 18: Synthetic and Biological Polymers47 Questions
Select questions type
An electron in the excited state does not necessarily return to a ground state in a single jump.
(True/False)
5.0/5  (33)
(33)
The atom size decreases as one proceeds from left to right across a period.
(True/False)
4.7/5  (33)
(33)
Match the following species with the corresponding electronic configuration from the list below.
Correct Answer:
Premises:
Responses:
(Matching)
4.8/5  (32)
(32)
For the electron of a hydrogen atom, which of the following quantum "jumps" requires the most energy?
(Multiple Choice)
4.9/5  (34)
(34)
Atoms in which of the following groups will likely lose three electrons?
(Multiple Choice)
4.8/5  (37)
(37)
If an element has a small electron affinity value it can easily lose an electron.
(True/False)
4.9/5  (32)
(32)
Write the complete electronic ground state configuration of the Se2- ion. Which other element (if any)shares this electronic configuration?
(Essay)
4.8/5  (38)
(38)
Atoms tend to increase in size as one moves from left to right across a period.
(True/False)
4.9/5  (38)
(38)
Which of the following elements is the largest size halogen?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following has attained a noble gas electronic configuration?
(Multiple Choice)
4.8/5  (28)
(28)
Should an oxygen atom (O)gain two electrons, it would then have the same ground state electronic configuration as an atom of neon (Ne), thus satisfying the octet rule.
(True/False)
4.8/5  (39)
(39)
Which color light is composed of photons having the largest energy?
(Multiple Choice)
4.9/5  (34)
(34)
A non-metal is an element that tends to gain valence electrons in chemical reactions becoming a cation in the process.
(True/False)
5.0/5  (46)
(46)
What is the trend in atomic size in the series of Group IIA elements Be through Ra?
(Multiple Choice)
4.8/5  (35)
(35)
Which is the correct electronic configuration for Se (atomic number 34)in the ground state?
(Multiple Choice)
4.9/5  (32)
(32)
Showing 61 - 80 of 97
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)