Exam 5: The Properties of Gases
Exam 1: The Quantum World100 Questions
Exam 2: Quantum Mechanics in Action: Atoms100 Questions
Exam 3: Chemical Bonds83 Questions
Exam 4: Molecular Shape and Structure95 Questions
Exam 5: The Properties of Gases94 Questions
Exam 6: Liquids and Solids95 Questions
Exam 7: Inorganic Materials100 Questions
Exam 8: Thermodynamics: The First Law94 Questions
Exam 9: Thermodynamics: The Second and Third Laws95 Questions
Exam 10: Physical Equilibria94 Questions
Exam 11: Chemical Equilibria94 Questions
Exam 12: Acids and Bases94 Questions
Exam 13: Aqueous Equilibria94 Questions
Exam 14: Electrochemistry94 Questions
Exam 15: Chemical Kinetics93 Questions
Exam 16: The Elements: the Main Group Elements189 Questions
Exam 17: The Elements: The D Block94 Questions
Exam 18: Nuclear Chemistry95 Questions
Exam 19: Organic Chemistry I94 Questions
Exam 20: Organic Chemistry II95 Questions
Select questions type
If the average speed of a carbon dioxide molecule is 410 m.s-1 at 25 C,what is the average speed of a molecule of methane at the same temperature?
(Multiple Choice)
4.9/5  (30)
(30)
How many liters of hydrogen gas measured at 25.0 C and 745 Torr are produced by detonation of 59.0 g of TNT,trinitrotoluene,C7H5(NO2)3?
(Multiple Choice)
4.8/5  (38)
(38)
Consider the following van der Waals coefficients: 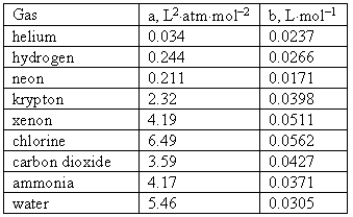 Which of the following gases has the largest attractive forces?
Which of the following gases has the largest attractive forces?
(Multiple Choice)
4.8/5  (46)
(46)
Which of the following gases will have the largest root mean square speed at 100 C?
(Multiple Choice)
4.8/5  (35)
(35)
The number of molecules in 44.8 L of nitrogen at exactly 0 C and 3.00 atm is
(Multiple Choice)
4.8/5  (34)
(34)
What is the root mean square speed of carbon dioxide molecules at 98 C?
(Multiple Choice)
4.9/5  (38)
(38)
The pressure at 20,000 feet above sea level is about 400.mmHg.This corresponds to
(Multiple Choice)
4.7/5  (39)
(39)
Sulfur dioxide reacts with oxygen gas to produce sulfur trioxide.What volume of oxygen gas will react with 15.0 L of sulfur dioxide if both gases are at 101.3 kPa and 125 C?
(Multiple Choice)
4.9/5  (42)
(42)
Consider the following statements:
1.Real gases act more like ideal gases as the temperature increases.
2.When n and T are constant, a decrease in P results in a decrease in V.
3.At 1 atm and 273 K, every molecule in a sample of a gas has the same speed.
4.At constant T, CO2 molecules at 1 atm and H2 molecules at 5 atm have the same
average kinetic energy.
Which of these statements is true?
(Multiple Choice)
4.8/5  (33)
(33)
What is the pressure of 800.g of nitrogen gas at 25.00°C at a volume of 5.0 L?
(Multiple Choice)
4.8/5  (40)
(40)
Consider two cylinders of gas,both with a volume of 25 L and at 1 atm and 25 C.If one cylinder contains nitrogen gas and the other argon,which of the following statements are true and which are false.
(a)The temperature of the gases is different.
(b)The average molecular speed of the gases is the same.
(c)The average kinetic energy of the gases is the same.
(Short Answer)
4.8/5  (44)
(44)
Lithium metal reacts with nitrogen gas to produce lithium nitride.What volume of nitrogen gas at 2.00 atm and 175 C is required to produce 75.0 g of lithium nitride?
(Multiple Choice)
4.8/5  (42)
(42)
A 0.479-g sample of nitrogen,oxygen or neon gas occupies a volume of 265 mL at 157 l Pa and 20.0 C.What are the molar mass and identity of the gas?
(Multiple Choice)
4.8/5  (36)
(36)
If 250.0 mL of a gas at STP weighs 2.00 g,what is the molar mass of the gas?
(Multiple Choice)
5.0/5  (39)
(39)
The density of citronellal,a mosquito repellant,is 1.45 g.L-1 at 365 C and 50.0 kPa.What is the molar mass of citronellal?
(Multiple Choice)
4.9/5  (35)
(35)
How many liters of nitrogen gas measured at 25.0 C and 745 Torr are produced by detonation of 90.8 g of TNT,trinitrotoluene,C7H5(NO2)3?
(Multiple Choice)
4.8/5  (37)
(37)
A 1.00-L sample of C2H4(g)at 2.00 atm and 293 K is burned in 8.00 L of oxygen gas at the same temperature and pressure to form carbon dioxide and water.If the reaction goes to completion,what is the final volume of all gases at 2.00 atm and 293 K?
(Multiple Choice)
4.7/5  (28)
(28)
Which molecules of the following gases will have the greatest average kinetic energy?
(Multiple Choice)
4.9/5  (37)
(37)
Showing 21 - 40 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)