Exam 16: The Elements: the Main Group Elements
Exam 1: The Quantum World100 Questions
Exam 2: Quantum Mechanics in Action: Atoms100 Questions
Exam 3: Chemical Bonds83 Questions
Exam 4: Molecular Shape and Structure95 Questions
Exam 5: The Properties of Gases94 Questions
Exam 6: Liquids and Solids95 Questions
Exam 7: Inorganic Materials100 Questions
Exam 8: Thermodynamics: The First Law94 Questions
Exam 9: Thermodynamics: The Second and Third Laws95 Questions
Exam 10: Physical Equilibria94 Questions
Exam 11: Chemical Equilibria94 Questions
Exam 12: Acids and Bases94 Questions
Exam 13: Aqueous Equilibria94 Questions
Exam 14: Electrochemistry94 Questions
Exam 15: Chemical Kinetics93 Questions
Exam 16: The Elements: the Main Group Elements189 Questions
Exam 17: The Elements: The D Block94 Questions
Exam 18: Nuclear Chemistry95 Questions
Exam 19: Organic Chemistry I94 Questions
Exam 20: Organic Chemistry II95 Questions
Select questions type
The molecule Al2Br6 is formed from two AlBr3 molecules.This reaction is
(Multiple Choice)
4.8/5  (34)
(34)
Xenon can exhibit all the following oxidation numbers in its compounds.
(Multiple Choice)
4.8/5  (25)
(25)
In ClF3,the number of regions of high electron concentration around the chlorine atom are
(Multiple Choice)
4.8/5  (35)
(35)
Consider the following possible reactions:
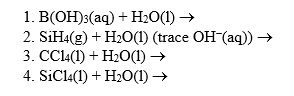 Which of the reactions that occur are Lewis acidbase reactions?
Which of the reactions that occur are Lewis acidbase reactions?
(Multiple Choice)
4.8/5  (44)
(44)
Consider the reaction
Fe(ClO4)3(aq)+ 6H2O(l) Fe(H2O)63+ + 3ClO4-(aq)
Which of the following statements are true?
(Multiple Choice)
4.8/5  (38)
(38)
The anhydride of phosphoric acid is produced by the reaction
(Multiple Choice)
4.8/5  (39)
(39)
Lithium and magnesium have similar properties and their compounds have covalent character.
(True/False)
4.9/5  (42)
(42)
The gaseous halides can all be synthesized by oxidation of their ions.
(True/False)
4.8/5  (38)
(38)
Showing 101 - 120 of 189
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)