Exam 23: Introduction to Organometallic Compounds
Exam 1: A Review of General Chemistry: Electrons, Bonds, and Molecular Properties191 Questions
Exam 2: Molecular Representations168 Questions
Exam 3: Acids and Bases127 Questions
Exam 4: Alkanes and Cycloalkanes116 Questions
Exam 5: Stereoisomerism141 Questions
Exam 6: Chemical Reactivity and Mechanisms96 Questions
Exam 7: Alkyl Halides: Nucleophilic Substitution and Elimination Reactions224 Questions
Exam 8: Addition Reactions of Alkenes153 Questions
Exam 9: Alkynes177 Questions
Exam 10: Radical Reactions102 Questions
Exam 11: Synthesis106 Questions
Exam 12: Alcohols and Phenols147 Questions
Exam 13: Ethers and Epoxides; Thiols and Sulfides139 Questions
Exam 14: Infrared Spectroscopy and Mass Spectrometry135 Questions
Exam 15: Nuclear Magnetic Resonance Spectroscopy140 Questions
Exam 16: Conjugated Pi Systems and Pericyclic Reactions140 Questions
Exam 17: Aromatic Compounds118 Questions
Exam 18: Aromatic Substitution Reactions122 Questions
Exam 19: Aldehydes and Ketones169 Questions
Exam 20: Carboxylic Acids and Their Derivatives144 Questions
Exam 21: Alpha Carbon Chemistry: Enols and Enolates147 Questions
Exam 22: Amines112 Questions
Exam 23: Introduction to Organometallic Compounds118 Questions
Exam 24: Carbohydrates144 Questions
Exam 25: Amino Acids, Peptides, and Proteins133 Questions
Exam 26: Lipids123 Questions
Exam 27: Synthetic Polymers119 Questions
Select questions type
A total synthesis of ingenol utilized the Corey-Posner/Whitesides-House coupling reaction in a key C-C bond forming step (J. Am. Chem. Soc. 2002, 124, 9726). Propose a reagent to perform this transformation. 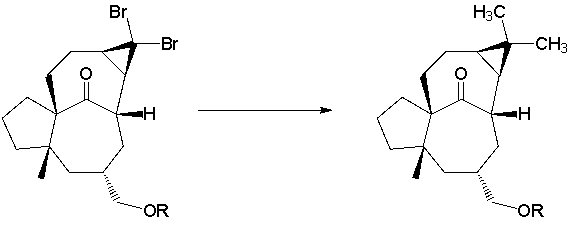
(Short Answer)
4.9/5  (42)
(42)
Which of the following electrophiles are commonly used with Gilman reagents to form C-C bonds?
(Multiple Choice)
4.8/5  (33)
(33)
Identify the product when an ester reacts with two equivalents of a Grignard reagent.
(Multiple Choice)
4.9/5  (43)
(43)
Which of the following are products of the reaction below? Select all that apply. 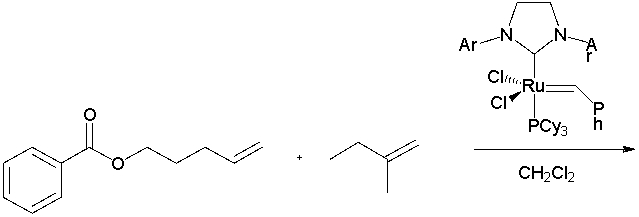
(Multiple Choice)
4.9/5  (53)
(53)
Which of the following methods is not a common/useful way to make an organozinc reagent?
(Multiple Choice)
4.9/5  (38)
(38)
What functional group most typically results when a Grignard Reagent reacts with an acid halide, after aqueous workup?
(Short Answer)
4.9/5  (37)
(37)
Which of the following is false regarding ring-opening metathesis?
(Multiple Choice)
4.8/5  (35)
(35)
Starting from bromobenzene and ________ as the only source of carbon atoms, 1-phenylcyclohexene can be synthesized using a Corey-Posner/Whitesides-House coupling reaction.
(Multiple Choice)
4.9/5  (39)
(39)
Macrocyle A was formed by a two- step process involving a hydroboration of an alkene in B followed by an intramolecular Suzuki coupling of a vinyl iodide in B to form the indicated bond below. What is the structure of B? (Org. Lett. 2002, 4, 2205). 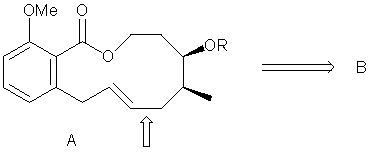
(Essay)
4.8/5  (38)
(38)
Identify the product when formaldehyde reacts with one equivalent of a Grignard reagent.
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following alkenes is most reactive in the Heck reaction? 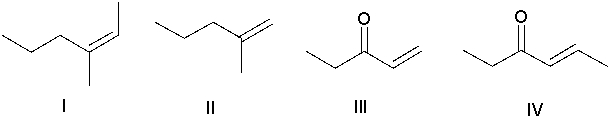
(Multiple Choice)
4.8/5  (29)
(29)
The reaction of a carbene with an alkene to make a cyclopropane commonly goes through which type mechanism?
(Multiple Choice)
4.8/5  (42)
(42)
Showing 41 - 60 of 118
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)