Exam 23: Introduction to Organometallic Compounds
Exam 1: A Review of General Chemistry: Electrons, Bonds, and Molecular Properties191 Questions
Exam 2: Molecular Representations168 Questions
Exam 3: Acids and Bases127 Questions
Exam 4: Alkanes and Cycloalkanes116 Questions
Exam 5: Stereoisomerism141 Questions
Exam 6: Chemical Reactivity and Mechanisms96 Questions
Exam 7: Alkyl Halides: Nucleophilic Substitution and Elimination Reactions224 Questions
Exam 8: Addition Reactions of Alkenes153 Questions
Exam 9: Alkynes177 Questions
Exam 10: Radical Reactions102 Questions
Exam 11: Synthesis106 Questions
Exam 12: Alcohols and Phenols147 Questions
Exam 13: Ethers and Epoxides; Thiols and Sulfides139 Questions
Exam 14: Infrared Spectroscopy and Mass Spectrometry135 Questions
Exam 15: Nuclear Magnetic Resonance Spectroscopy140 Questions
Exam 16: Conjugated Pi Systems and Pericyclic Reactions140 Questions
Exam 17: Aromatic Compounds118 Questions
Exam 18: Aromatic Substitution Reactions122 Questions
Exam 19: Aldehydes and Ketones169 Questions
Exam 20: Carboxylic Acids and Their Derivatives144 Questions
Exam 21: Alpha Carbon Chemistry: Enols and Enolates147 Questions
Exam 22: Amines112 Questions
Exam 23: Introduction to Organometallic Compounds118 Questions
Exam 24: Carbohydrates144 Questions
Exam 25: Amino Acids, Peptides, and Proteins133 Questions
Exam 26: Lipids123 Questions
Exam 27: Synthetic Polymers119 Questions
Select questions type
Gillman reagents are intolerant to the presence of what functional group?
(Multiple Choice)
4.7/5  (29)
(29)
What functional group typically results when an organocopper compound (lithium organocuprate) reacts with an acid chloride?
(Short Answer)
4.9/5  (39)
(39)
The Stille reaction involves coupling of which pair of reagents?
(Multiple Choice)
4.8/5  (36)
(36)
Starting from vinyl chloride and ________ as the only source of carbon atoms, 1-heptene can be synthesized using a Corey-Posner/Whitesides-House coupling reaction.
(Multiple Choice)
4.9/5  (33)
(33)
Identify the product when ethylene oxide reacts with one equivalent of a Grignard reagent.
(Multiple Choice)
4.8/5  (37)
(37)
Diethyl ether is a good solvent to be used with Grignard reagents. Which of the following explanations for this fact is false?
(Multiple Choice)
4.7/5  (38)
(38)
The following synthesis is not likely to work. Which of the following explains why? 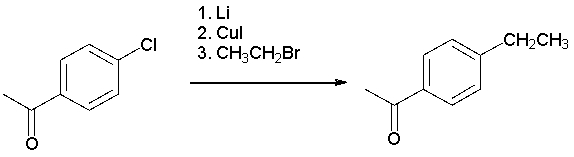
(Multiple Choice)
4.8/5  (38)
(38)
What is the product of treating styrene with the Grubbs Catalyst?
(Multiple Choice)
4.8/5  (41)
(41)
A total synthesis of ingenol utilized a dibromocyclopropanation via a carbene intermediate (J. Am. Chem. Soc. 2002, 124, 9726). What key type of reagent is missing from the reaction conditions? 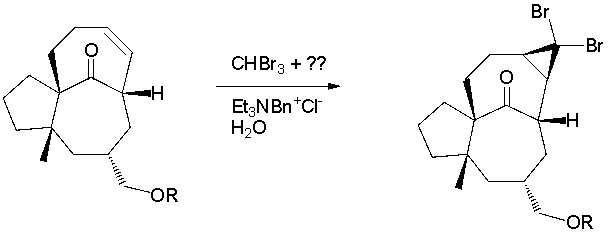
(Multiple Choice)
4.8/5  (37)
(37)
What is the product of the following ring-opening/ring-closing metathesis reaction? (J. Am. Chem. Soc. 1996, 118, 6634.) 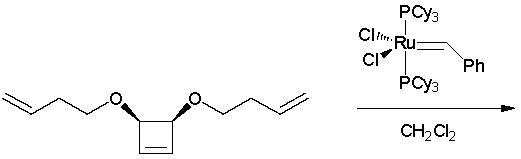
(Essay)
4.7/5  (34)
(34)
Which of the following solvents is/are good solvents to be used in the preparation of organometallic reagents such as organolithiums and Grignard reagents? Select all that apply.
(Multiple Choice)
4.7/5  (29)
(29)
The final steps of Sammakia's total synthesis of caerulomycin C involved the reaction below (Org. Lett. 2002, 4, 2385). Draw the product. 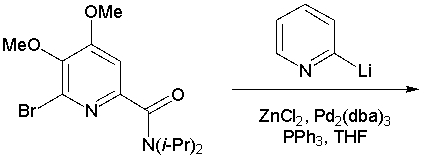
(Essay)
4.9/5  (32)
(32)
What diene is the reactant in the following ring-closing metathesis reaction? 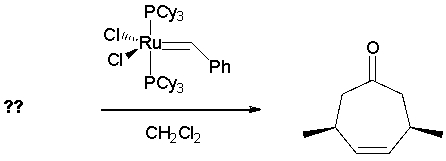
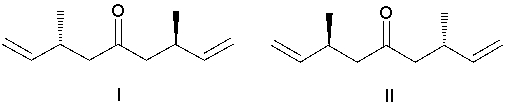
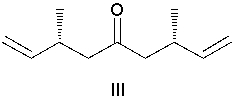
(Multiple Choice)
4.8/5  (34)
(34)
Showing 101 - 118 of 118
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)