Exam 8: Alkenes and Alkynes Ii: Addition Reactions
Exam 1: Carbon Compounds and Chemical Bonds134 Questions
Exam 2: Representative Carbon Compounds: Functional Groups, Intermolecular Forces, and Infrared Ir Spectroscopy114 Questions
Exam 3: An Introduction to Organic Reactions: Acids and Bases47 Questions
Exam 4: Alkanes: Nomenclature, Conformational Analysis, and an Introduction to Synthesis125 Questions
Exam 5: Stereochemistry: Chiral Molecules150 Questions
Exam 6: Ionic Reactions - Nucleophilic Substitution and Elimination Reactions of Alkyl Halides146 Questions
Exam 7: Alkenes and Alkynes I: Properties and Synthesis, Elimination Reactions of Alkyl Halides99 Questions
Exam 8: Alkenes and Alkynes Ii: Addition Reactions140 Questions
Exam 9: Nuclear Magnetic Resonance and Mass Spectrometry: Tools for Structure Determination94 Questions
Exam 10: Radical Reactions114 Questions
Exam 11: Alcohols and Ethers172 Questions
Exam 12: Alcohols From Carbonyl Compounds Oxidation-Reduction and Organometallic Compounds147 Questions
Exam 13: Conjugated Unsaturated Systems166 Questions
Exam 14: Aromatic Compounds151 Questions
Exam 15: Reactions of Aromatic Compounds173 Questions
Exam 16: Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group165 Questions
Exam 17: Aldehydes and Ketones Ii Aldol Reactions131 Questions
Exam 18: Carboxylic Acids and Their Derivatives Nucleophilic Addition - Elimination at the Acyl Carbon124 Questions
Exam 19: Synthesis and Reactions of Beta-Dicarbonyl Compounds: More Chemistry of Enolate Ions131 Questions
Exam 20: Amines148 Questions
Exam 21: Phenols and Aryl Halides: Nucleophilic Aromatic Substitution87 Questions
Exam 22: Carbohydrates104 Questions
Exam 23: Lipids99 Questions
Exam 24: Amino Acids and Proteins94 Questions
Exam 25: Nucleic Acids and Protein Synthesis89 Questions
Select questions type
Which of these compounds will react with cold concd.H2SO4,as well as Br2 in CCl4 ?
(Multiple Choice)
4.7/5  (43)
(43)
Acid-catalyzed hydration of 2-methyl-1-butene would yield which of the following?
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following reactions of cyclobutene would yield a meso product?
(Multiple Choice)
4.7/5  (41)
(41)
What is the major product of the following reaction sequence? 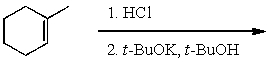
(Multiple Choice)
4.9/5  (31)
(31)
Draw Fischer projection formulas of the major product of the reaction between E-2-methyl-3-hexene and aqueous Br2.
(Essay)
4.8/5  (40)
(40)
What is the major product of the following reaction sequence? 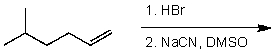
(Multiple Choice)
4.7/5  (28)
(28)
The reaction of BrCl (bromine monochloride)with 1-methylcyclopentene will produce as the predominant product: 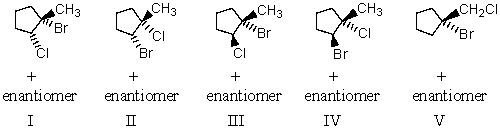
(Multiple Choice)
4.8/5  (36)
(36)
The interaction of the bond of an alkene with an electrophile can initially result in the formation of a species termed a complex.Which of these cannot combine with an alkene to form a complex?
(Multiple Choice)
4.7/5  (35)
(35)
An unknown compound,A,has the molecular formula C7H12.On oxidation with hot aqueous potassium permanganate,A yields CH3CH2COOH and (CH3)2CHCOOH.Which of the following structures best represents A?
(Multiple Choice)
4.9/5  (34)
(34)
One mole of an optically active compound,X,with the molecular formula C6H8 reacts with three moles of hydrogen in the presence of a catalyst to yield an optically inactive product that cannot be resolved.X also exhibits IR absorption at approximately 3300 cm-1.Which is a possible structure for X?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following could be used as the basis for a simple test that would distinguish between 1-pentyne and pentane?
(Multiple Choice)
4.9/5  (35)
(35)
What is the major product of the following reaction sequence? 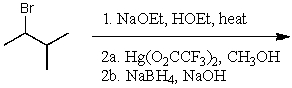
(Multiple Choice)
4.9/5  (37)
(37)
(R)-3-Chloro-1-butene reacts with HCl by Markovnikov addition,and the products are separated by gas chromatography.How many total fractions would be obtained and how many would be optically active?
(Multiple Choice)
4.9/5  (42)
(42)
What is the chief product of the reaction of IBr with 2-methyl-2-pentene?
(Multiple Choice)
4.8/5  (36)
(36)
An alkene with the molecular formula C10H18 is treated with ozone and then with zinc and acetic acid.The only product isolated from these reactions is: 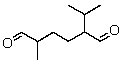 What is the structure of the alkene?
What is the structure of the alkene? 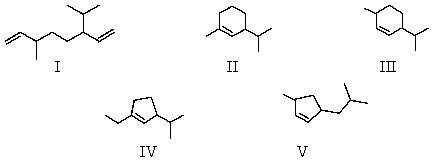
(Multiple Choice)
4.8/5  (37)
(37)
Showing 121 - 140 of 140
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)