Exam 9: Nuclear Magnetic Resonance and Mass Spectrometry: Tools for Structure Determination
Exam 1: Carbon Compounds and Chemical Bonds134 Questions
Exam 2: Representative Carbon Compounds: Functional Groups, Intermolecular Forces, and Infrared Ir Spectroscopy114 Questions
Exam 3: An Introduction to Organic Reactions: Acids and Bases47 Questions
Exam 4: Alkanes: Nomenclature, Conformational Analysis, and an Introduction to Synthesis125 Questions
Exam 5: Stereochemistry: Chiral Molecules150 Questions
Exam 6: Ionic Reactions - Nucleophilic Substitution and Elimination Reactions of Alkyl Halides146 Questions
Exam 7: Alkenes and Alkynes I: Properties and Synthesis, Elimination Reactions of Alkyl Halides99 Questions
Exam 8: Alkenes and Alkynes Ii: Addition Reactions140 Questions
Exam 9: Nuclear Magnetic Resonance and Mass Spectrometry: Tools for Structure Determination94 Questions
Exam 10: Radical Reactions114 Questions
Exam 11: Alcohols and Ethers172 Questions
Exam 12: Alcohols From Carbonyl Compounds Oxidation-Reduction and Organometallic Compounds147 Questions
Exam 13: Conjugated Unsaturated Systems166 Questions
Exam 14: Aromatic Compounds151 Questions
Exam 15: Reactions of Aromatic Compounds173 Questions
Exam 16: Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group165 Questions
Exam 17: Aldehydes and Ketones Ii Aldol Reactions131 Questions
Exam 18: Carboxylic Acids and Their Derivatives Nucleophilic Addition - Elimination at the Acyl Carbon124 Questions
Exam 19: Synthesis and Reactions of Beta-Dicarbonyl Compounds: More Chemistry of Enolate Ions131 Questions
Exam 20: Amines148 Questions
Exam 21: Phenols and Aryl Halides: Nucleophilic Aromatic Substitution87 Questions
Exam 22: Carbohydrates104 Questions
Exam 23: Lipids99 Questions
Exam 24: Amino Acids and Proteins94 Questions
Exam 25: Nucleic Acids and Protein Synthesis89 Questions
Select questions type
A prominent (M 1+ -18)peak suggests that the compound might be a(n):
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
D
Which one of the following best represents the predicted approximate chemical shift and coupling for the hydrogen(s)indicated with the arrow? 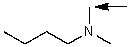
Free
(Multiple Choice)
4.9/5  (45)
(45)
Correct Answer:
C
Determine the most likely structure of a compound,with the molecular formula C9H12,which gave a 1H NMR spectrum consisting of: a doublet at 1.25
A septet at 2.90 and
A multiplet at 7.25 ![Determine the most likely structure of a compound,with the molecular formula C<sub>9</sub>H<sub>12</sub>,which gave a <sup>1</sup>H NMR spectrum consisting of: a doublet at \delta 1.25 A septet at \delta 2.90 and A multiplet at \delta 7.25 ]](https://storage.examlex.com/TB5901/11ea9a02_1b24_5831_8bb6_315348c46757_TB5901_00.jpg) ]
]
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
D
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C9H14O? IR data shows no characteristic peak around 1700 cm-1.The 13C-NMR chemical shifts (ppm): 108.4,50.9,31.6,23.5,2.0.Relative integration is known. 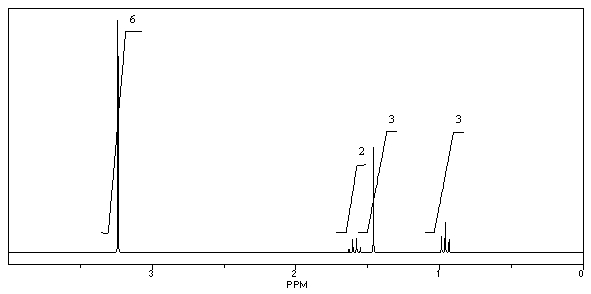
(Multiple Choice)
4.8/5  (28)
(28)
A compound with the molecular formula C6H15N gave the following 1H NMR spectrum: triplet, 0.90
Quartet, 2.4
There were no other signals.The most likely structure for the compound is:
(Multiple Choice)
4.8/5  (30)
(30)
The data below from the molecular ion region of the mass spectrum of a halogen-containing compound are consistent with the presence of what halogen(s)in the original compound? intensity
M +
51)0
M + +2
100)0
M + +4
49)0
(Multiple Choice)
4.9/5  (29)
(29)
For the C2 methylene group in 1-bromopropane,the theoretical multiplicity in the 1H NMR spectrum,presuming that Jab is sufficiently different from Jbc and that the instrument has sufficient resolving power,is which of these? 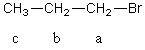
(Multiple Choice)
4.9/5  (38)
(38)
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C6H12O2? Relative integration is shown. 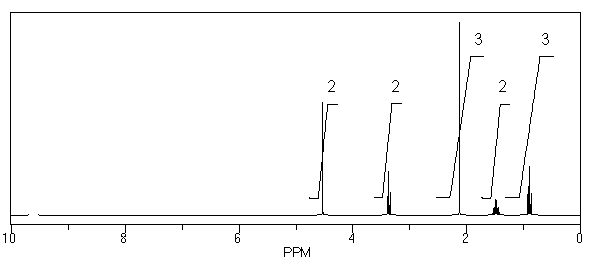
(Multiple Choice)
4.8/5  (47)
(47)
Which one of the following best represents the predicted approximate chemical shift and coupling for the hydrogen(s)indicated with the arrow? 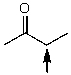
(Multiple Choice)
5.0/5  (47)
(47)
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C7H12O4? The 13C-NMR spectrum shows peaks at 14.1,40.8,61.0 and 166.8 ppm.Relative integration is shown. 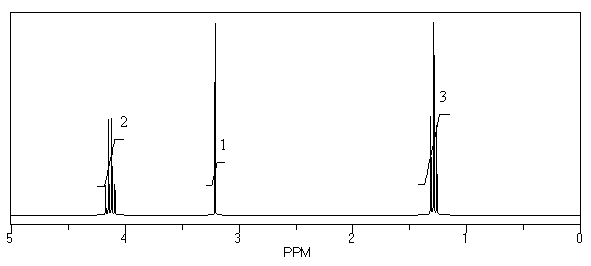
(Multiple Choice)
4.8/5  (35)
(35)
The 1H NMR spectrum of which of the compounds below,all of formula C7H12O2,would consist of three singlets only? 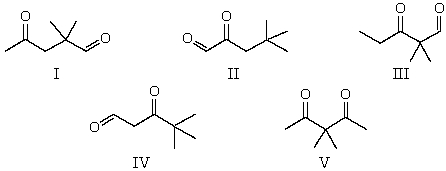
(Multiple Choice)
4.7/5  (39)
(39)
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C5H8O? The IR spectrum does show a characteristic stretch around 1700 cm-1.Relative integration is shown. 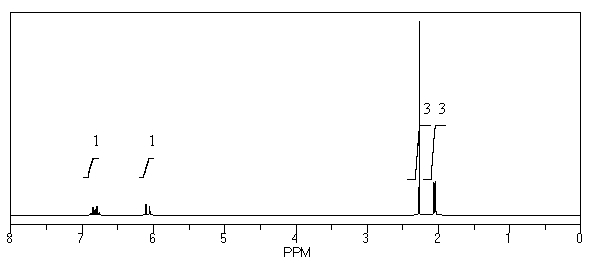
(Multiple Choice)
4.8/5  (36)
(36)
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C6H11N? In the IR spectrum you notice a stretch at about 2250 cm-1.Relative integration is shown. 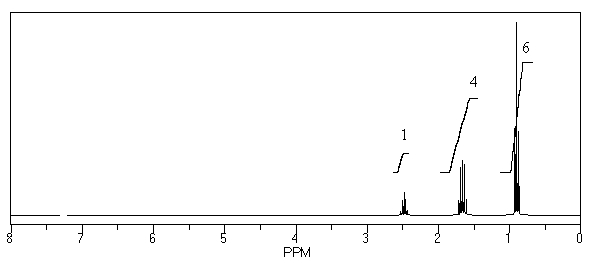
(Multiple Choice)
4.9/5  (44)
(44)
The C7 compound which gives 3 signals in the broadband proton-decoupled 13C spectrum could be:
(Multiple Choice)
4.8/5  (31)
(31)
A compound with the molecular formula C4H10O gives a 1H NMR spectrum consisting only of a quartet centered at 3.5 and a triplet at 1.1.The most likely structure for the compound is:
(Multiple Choice)
4.9/5  (32)
(32)
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C6H10O2? Relative integration is shown. 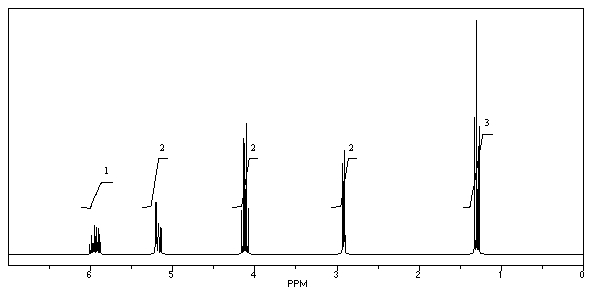
(Multiple Choice)
4.8/5  (43)
(43)
Showing 1 - 20 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)