Exam 18: Heat and the First Law of Thermodynamics
Exam 1: Systems of Measurement86 Questions
Exam 2: Motion in One Dimension103 Questions
Exam 3: Motion in Two and Three Dimensions67 Questions
Exam 4: Newtons Laws117 Questions
Exam 5: Applications of Newtons Laws75 Questions
Exam 6: Work and Energy71 Questions
Exam 7: Conservation of Energy73 Questions
Exam 8: Systems of Particles and Conservation of Linear Momentum107 Questions
Exam 9: Rotation119 Questions
Exam 10: Conservation of Angular Momentum67 Questions
Exam 11: Gravity90 Questions
Exam 12: Static Equilibrium and Elasticity65 Questions
Exam 13: Fluids91 Questions
Exam 14: Oscillations138 Questions
Exam 15: Wave Motion122 Questions
Exam 16: Superposition and Standing Waves125 Questions
Exam 17: Temperature and the Kinetic Theory of Gases85 Questions
Exam 18: Heat and the First Law of Thermodynamics114 Questions
Exam 19: The Second Law of Thermodynamics61 Questions
Exam 20: Thermal Properties and Processes54 Questions
Select questions type
A state variable is one that allows other variables to be determined using a relationship. Which of the following variables are state variables?
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
D
A small water reactor recently installed at Podunk College is operating at the boiling point of water due to the malfunctioning of the cooling system. The operators observe that the water boils away at the rate of 10 L/min. If they assume that all of the reactor energy is absorbed in the water, the power developed by the reactor is approximately
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
D
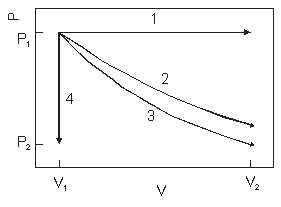 The diagram above show the state of an ideal gas going from (V1, P1) to a final state. Which path best represents an isothermal expansion?
The diagram above show the state of an ideal gas going from (V1, P1) to a final state. Which path best represents an isothermal expansion?
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
B
The molar heat capacity at constant volume of a gas is found to be 20.74 J/mol · K. What is the molar heat capacity at constant pressure of this gas? (The ideal-gas law constant is R = 8.31 J/mol · K)
(Multiple Choice)
4.8/5  (33)
(33)
You add 50 g of ice cubes to 125 g of water that is initially at 20ºC in a calorimeter of negligible heat capacity. When the system has reached equilibrium, how much of the ice remains?
(Multiple Choice)
4.7/5  (28)
(28)
If 100 g of steam at 100 C were mixed with 10 kg of ice at -100 C, find the final temperature of the mixture assuming no heat losses to the surroundings.
(Multiple Choice)
4.8/5  (37)
(37)
A 6.0-g lead bullet traveling at 300 m/s penetrates a wooden block and stops. If 50 percent of the initial kinetic energy of the bullet is converted into thermal energy in the bullet, by how much does the bullet's temperature increase? (The specific heat of lead is 128 J/kg · K.)
(Multiple Choice)
4.8/5  (33)
(33)
A cylinder contains 20 L of air at 1 atm. The ratio of Cp to CV for air is 1.41. If this sample of air is compressed adiabatically to a volume of 5 L, the pressure after compression is approximately
(Multiple Choice)
4.8/5  (28)
(28)
In an adiabatic reversible compression of an ideal gas, there is a decrease in
(Multiple Choice)
4.7/5  (34)
(34)
Use the following to answer question : 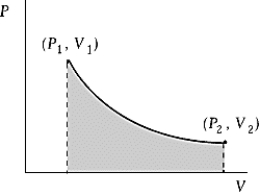 -An ideal gas initially at 100ºC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L. It undergoes a quasistatic, isothermal expansion until its pressure is reduced to 150 kPa. How much heat enters the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
-An ideal gas initially at 100ºC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L. It undergoes a quasistatic, isothermal expansion until its pressure is reduced to 150 kPa. How much heat enters the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
(Multiple Choice)
5.0/5  (38)
(38)
In a certain thermodynamic process, 20 cal of heat are removed from a system and 30 cal of work are done on the system. The internal energy of the system
(Multiple Choice)
4.8/5  (36)
(36)
The equation of state for a certain gas under isothermal conditions is
PV = 31.2,
Where the units are SI. The work done by this gas as its volume increases isothermally from 1 L to 10 L is approximately
(Multiple Choice)
4.8/5  (29)
(29)
Two liquids, A and B, are mixed together, and the resulting temperature is 22 C. If liquid A has mass m and was initially at temperature 35 C, and liquid B has mass 3m and was initially at temperature 11 C, calculate the ratio of the specific heats of A divided by B.
(Multiple Choice)
4.9/5  (44)
(44)
Use the following to answer question : 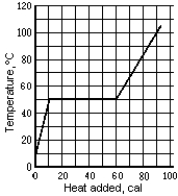 -The graph shows the temperature of a 1.0-g sample of material as heat is added to it. The material is initially a solid at 10ºC. The pressure remains constant, and there is no chemical change. The specific heat of the liquid phase is
-The graph shows the temperature of a 1.0-g sample of material as heat is added to it. The material is initially a solid at 10ºC. The pressure remains constant, and there is no chemical change. The specific heat of the liquid phase is
(Multiple Choice)
4.9/5  (31)
(31)
A 4-kg mass of metal of unknown specific heat at a temperature of 600 C is dropped into 0.5 kg of ice and 0.5 kg of water both at 0 C. With no heat losses to the surroundings, the equilibrium temperature of the mixture is 85 C. Calculate the specific heat of the metal.
(Multiple Choice)
4.9/5  (35)
(35)
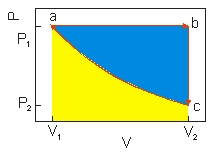 An ideal gas undergoes a cyclic expansion and compression along the path a b c a as shown above. The work done along b c of the cycle is the area
An ideal gas undergoes a cyclic expansion and compression along the path a b c a as shown above. The work done along b c of the cycle is the area
(Multiple Choice)
4.9/5  (36)
(36)
The equation of state for a certain gas under isothermal conditions is
PV = 31.2,
Where the units are SI. The work done by this gas as its volume increases isothermally from 0.2 m3 to 0.8 m3 is approximately
(Multiple Choice)
4.9/5  (37)
(37)
Two liquids, A and B, are mixed together. Liquid A has mass m and was initially at temperature 40 C, and liquid B has mass 2m and was initially at temperature 5 C. The specific heat of liquid A is 1.5 times that of liquid B.
Calculate the final temperature of the mixture.
(Multiple Choice)
4.9/5  (34)
(34)
Use the following to answer question : 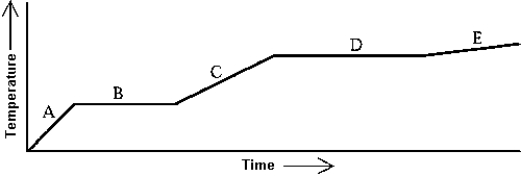 -Heat is added to a substance at a constant rate. The substance starts as a solid and is melted; the liquid is heated and vaporized; finally, the vapor is heated. This process is shown in the graph. The specific heat of the liquid can be found by
-Heat is added to a substance at a constant rate. The substance starts as a solid and is melted; the liquid is heated and vaporized; finally, the vapor is heated. This process is shown in the graph. The specific heat of the liquid can be found by
(Multiple Choice)
4.9/5  (37)
(37)
The work done by an ideal gas in an isothermal expansion from volume V1 to volume V2 is given by the formula:
W = nRT ln(V2/V1)
Standard atmospheric pressure (1 atm) is 101.3 kPa. If 1.0 L of He gas at room temperature (20ºC) and 1.0 atm of pressure is compressed isothermally to a volume of 100 mL, how much work is done on the gas?
(Multiple Choice)
4.9/5  (27)
(27)
Showing 1 - 20 of 114
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)