Exam 17: Temperature and the Kinetic Theory of Gases
Exam 1: Systems of Measurement86 Questions
Exam 2: Motion in One Dimension103 Questions
Exam 3: Motion in Two and Three Dimensions67 Questions
Exam 4: Newtons Laws117 Questions
Exam 5: Applications of Newtons Laws75 Questions
Exam 6: Work and Energy71 Questions
Exam 7: Conservation of Energy73 Questions
Exam 8: Systems of Particles and Conservation of Linear Momentum107 Questions
Exam 9: Rotation119 Questions
Exam 10: Conservation of Angular Momentum67 Questions
Exam 11: Gravity90 Questions
Exam 12: Static Equilibrium and Elasticity65 Questions
Exam 13: Fluids91 Questions
Exam 14: Oscillations138 Questions
Exam 15: Wave Motion122 Questions
Exam 16: Superposition and Standing Waves125 Questions
Exam 17: Temperature and the Kinetic Theory of Gases85 Questions
Exam 18: Heat and the First Law of Thermodynamics114 Questions
Exam 19: The Second Law of Thermodynamics61 Questions
Exam 20: Thermal Properties and Processes54 Questions
Select questions type
If the pressure and volume of an ideal gas are both reduced to half their original value, the absolute temperature of the gas is
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
E
A large balloon is being filled with He from gas cylinders. The temperature is 25 C and the pressure is 1 atmosphere. The volume of the inflated balloon is 2500 m3. What was the volume of He in the cylinders if the gas was under a pressure of 110 atmospheres and at a temperature of 12 C when in the gas cylinders?
Free
(Multiple Choice)
5.0/5  (33)
(33)
Correct Answer:
B
A room measures 3 m 4 m 2 m and is at 15 C and 1 atm. Assuming that it only has the two diatomic gases, N2 and O2, how much heat is needed to increase the temperature to 25 C? (Ignore the loss in air as the temperature heats up.)
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
D
Which of the following statements about thermal contact and thermal equilibrium is NOT true?
(Multiple Choice)
4.8/5  (40)
(40)
Ethyl chloride (C2H5Cl) is used as a refrigerant under certain circumstances. One mole of this substance contains Avogadro's number of
(Multiple Choice)
4.8/5  (38)
(38)
A hailstorm causes an average pressure of 1.4 N/m2 on the 200-m2 flat roof of a house. The hailstones, each of mass 7.0 10-3 kg, have an average velocity of 10 m/s perpendicular to the roof and rebound after hitting the roof with the same speed. How many hailstones hit the roof each second?
(Multiple Choice)
4.7/5  (38)
(38)
At what Kelvin temperature does the rms speed of the oxygen (O2) molecules in the air near the surface of the earth become equal to the escape speed from the earth? (R = 8.31 J/mol · K; molar mass of O2 gas is 32 g/mol; radius of the earth RE = 6.37 106 m; the escape speed from the earth is 11.2 km/s)
(Multiple Choice)
4.9/5  (30)
(30)
If you plot a graph with Fahrenheit temperatures along the horizontal axis and the corresponding Celsius temperatures along the vertical axis, the slope of the equal-temperature line will be
(Multiple Choice)
4.8/5  (31)
(31)
Assume that helium is a perfect gas and that the volume of a cylinder containing helium is independent of temperature. A cylinder of helium at +85ºC has a pressure of 208 atm. The pressure of the helium when it is cooled to -55ºC is
(Multiple Choice)
4.8/5  (33)
(33)
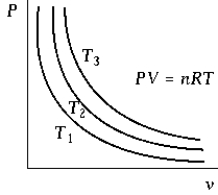 The isotherm that corresponds to the lowest temperature is the one labeled
The isotherm that corresponds to the lowest temperature is the one labeled
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following is an assumption that is made in the kinetic theory of gases?
(Multiple Choice)
4.9/5  (29)
(29)
The rms speed of oxygen molecules is 460 m/s at 0ºC. The molecular weight of oxygen is 8 times the molecular weight of helium. The rms speed of helium at 40ºC is approximately
(Multiple Choice)
4.9/5  (38)
(38)
Use the following to answer question: 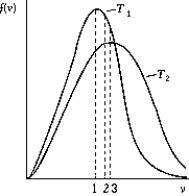 -The figure shows the distribution of the molecular speeds of a gas for two different temperatures.
-The figure shows the distribution of the molecular speeds of a gas for two different temperatures.
(Multiple Choice)
4.9/5  (34)
(34)
A rigid container of air is at atmospheric pressure and 27ºC. To double the pressure in the container, heat it to
(Multiple Choice)
4.8/5  (36)
(36)
Two monoatomic gases, helium and neon, are mixed in the ratio of 2 to 1 and are in thermal equilibrium at temperature T (the molar mass of neon = 5 the molar mass of helium). If the average kinetic energy of each helium atom is U, calculate the average energy of each neon atom.
(Multiple Choice)
4.9/5  (32)
(32)
A volume of an ideal gas goes through a temperature change from 20ºC to 60ºC. The relation between the average molecular kinetic energy at 20ºC (K1) and that at 60ºC (K2) is
(Multiple Choice)
4.7/5  (30)
(30)
If the rms speed of nitrogen molecules (molecular weight of N2 is 28 g/mol) at 273 K is 492 m/s, the rms speed of oxygen molecules (molecular weight of O2 is 32 g/mol) at the same temperature is approximately
(Multiple Choice)
4.8/5  (39)
(39)
A cylinder of volume 50 L contains oxygen gas at a pressure of 2 atm. If nitrogen gas of volume 25 L and at pressure 1 atm is added to the oxygen cylinder, the new pressure is
(Multiple Choice)
4.9/5  (41)
(41)
Showing 1 - 20 of 85
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)