Exam 21: Transition Metal Complexes
Exam 1: The Basics158 Questions
Exam 2: Families of Carbon151 Questions
Exam 3: Acids and Bases149 Questions
Exam 4: Nomenclature and Conformations of Alkanes and Cycloalkanes163 Questions
Exam 5: Stereochemistry179 Questions
Exam 6: Nucleophilic Reactions91 Questions
Exam 7: Alkenes and Alkynes I220 Questions
Exam 8: Alkenes and Alkynes II163 Questions
Exam 9: Nuclear Magnetic Resonance and Mass Spectrometry158 Questions
Exam 10: Radical Reactions148 Questions
Exam 11: Alcohols and Ethers256 Questions
Exam 12: Alcohols From Carbonyl Compounds210 Questions
Exam 13: Conjugated Unsaturated Systems201 Questions
Exam 14: Aromatic Compounds186 Questions
Exam 15: Reactions of Aromatic Compounds207 Questions
Exam 16: Aldehydes and Ketones189 Questions
Exam 17: Carboxylic Acids and Their Derivatives213 Questions
Exam 18: Reactions at the Α Carbon of Carbonyl Compounds190 Questions
Exam 19: Condensation and Conjugate Addition Reactions of Carbonyl Compounds182 Questions
Exam 20: Amines207 Questions
Exam 21: Transition Metal Complexes86 Questions
Exam 22: Carbohydrates124 Questions
Exam 23: Lipids127 Questions
Exam 24: Amino Acids and Proteins135 Questions
Exam 25: Nucleic Acids and Protein Synthesis126 Questions
Select questions type
Consider the following product that was made from a cross-coupling reaction. 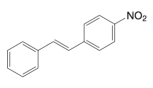 This product could have been made from
This product could have been made from 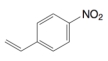 and ____.
and ____.
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
B
Coupling an aryl borate with an aryl halide,using a Pd catalyst,is an example of ____.
Free
(Multiple Choice)
4.9/5  (40)
(40)
Correct Answer:
B
Reacting 1-butyne with iodobenzene in the presence of CuI,Pd catalyst (in an amine base)will generate ____ as its product.
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
C
A student wants to make 4-nitro-1-phenyl-1-butyne using Sonogahira coupling conditions,beginning with iodobenzene.This student will also require ____ in order to make this product.
(Short Answer)
4.9/5  (28)
(28)
Consider the transition metal complex FeCp(CO)3+.The oxidation state of the metal is ____.
(Multiple Choice)
4.7/5  (36)
(36)
An alkanide anion donates a total of ____ electrons to a metal complex.
(Short Answer)
4.7/5  (38)
(38)
Consider the transition metal complex Pd(PPh3)4.The oxidation state of the metal is ____.
(Multiple Choice)
4.9/5  (40)
(40)
Treating 1-octene with Wilkinson's catalyst will produce ____.
(Multiple Choice)
4.7/5  (37)
(37)
Rh(NH3)ClH2 decomposing to Rh(NH3)3Cl and H2(g)is an example of a(n)_____ reaction.
(Multiple Choice)
4.8/5  (37)
(37)
Reacting propyne with iodobenzene in the presence of CuI,Pd catalyst (in an amine base)will generate ____ as its product.
(Multiple Choice)
4.9/5  (38)
(38)
Consider the transition metal complex FeCp2.The oxidation state of the metal is ____.
(Multiple Choice)
4.8/5  (35)
(35)
Reacting 3-nitrostyrene with iodobenzene under Heck-Mizoroki conditions will generate ____ as its product.
(Multiple Choice)
4.8/5  (39)
(39)
A student wants to make 3-chloro-1-phenyl-1-propyne using Sonogahira coupling conditions,beginning with iodobenzene.This student will also require ____ in order to make this product.
(Short Answer)
4.9/5  (40)
(40)
The last step in the mechanism of an alkene reacting with Wilkinson's catalyst is ____.
(Multiple Choice)
4.7/5  (32)
(32)
Organometallic complexes,such as Grignard reagents,can form ____ bonds.
(Short Answer)
4.9/5  (44)
(44)
Consider the transition metal complex FeCp2.The total number of valence electrons this complex possesses is ____.
(Multiple Choice)
4.9/5  (37)
(37)
Consider the transition metal complex CrOF5.The oxidation state of the metal is ____.
(Multiple Choice)
4.9/5  (43)
(43)
The transition metal found in the core of Vitamin B12 is ____ and in its stable commercial form has a ____ oxidation state.
(Multiple Choice)
4.8/5  (32)
(32)
Consider the transition metal complex Rh(PPh3)3Cl.The oxidation state of the metal is ____.
(Multiple Choice)
4.9/5  (37)
(37)
Showing 1 - 20 of 86
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)