Exam 7: Solutions and Colloids
Exam 1: Matter, measurements, and Calculations92 Questions
Exam 2: Atoms and Molecules89 Questions
Exam 3: Electronic Structure and the Periodic Law86 Questions
Exam 4: Forces Between Particles89 Questions
Exam 5: Chemical Reactions88 Questions
Exam 6: The States of Matter88 Questions
Exam 7: Solutions and Colloids87 Questions
Exam 8: Reaction Rates and Equilibrium86 Questions
Exam 9: Acids, bases, and Salts86 Questions
Exam 10: Radioactivity and Nuclear Processes86 Questions
Exam 11: Organic Compounds: Alkanes87 Questions
Exam 12: Unsaturated Hydrocarbons88 Questions
Exam 13: Alcohols, phenols, and Ethers86 Questions
Exam 14: Aldehydes and Ketones86 Questions
Exam 15: Carboxylic Acids and Esters86 Questions
Exam 16: Amines and Amides85 Questions
Exam 17: Carbohydrates88 Questions
Exam 18: Lipids91 Questions
Exam 19: Proteins89 Questions
Exam 20: Enzymes88 Questions
Exam 21: Nucleic Acids and Protein Synthesis90 Questions
Exam 22: Nutrition and Energy for Life89 Questions
Exam 23: Carbohydrate Metabolism90 Questions
Exam 24: Lipid and Amino Acid Metabolism94 Questions
Exam 25: Body Fluids86 Questions
Select questions type
An ion in solution that is surrounded by water is a(n)____ ion.
(Multiple Choice)
4.8/5  (31)
(31)
Express the following concentration of solution in terms of molarity: 3.00 L of solution contains 1.75 mol of solute.
(Multiple Choice)
4.8/5  (30)
(30)
When sodium acetate dissolves in water,the following reaction occurs. 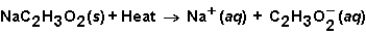 This solution could be used
This solution could be used
(Multiple Choice)
5.0/5  (42)
(42)
How many mL of 6.00 M HCl are needed to prepare 1500 mL of 0.200 M HCl solution?
(Multiple Choice)
4.8/5  (30)
(30)
Attractive forces between solute and solvent molecules are an important factor in solution formation.
(True/False)
4.9/5  (48)
(48)
Solvents and hydrated ions can usually pass though dialyzing membranes
(True/False)
4.7/5  (41)
(41)
Showing 81 - 87 of 87
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)