Exam 7: Solutions and Colloids
Exam 1: Matter, measurements, and Calculations92 Questions
Exam 2: Atoms and Molecules89 Questions
Exam 3: Electronic Structure and the Periodic Law86 Questions
Exam 4: Forces Between Particles89 Questions
Exam 5: Chemical Reactions88 Questions
Exam 6: The States of Matter88 Questions
Exam 7: Solutions and Colloids87 Questions
Exam 8: Reaction Rates and Equilibrium86 Questions
Exam 9: Acids, bases, and Salts86 Questions
Exam 10: Radioactivity and Nuclear Processes86 Questions
Exam 11: Organic Compounds: Alkanes87 Questions
Exam 12: Unsaturated Hydrocarbons88 Questions
Exam 13: Alcohols, phenols, and Ethers86 Questions
Exam 14: Aldehydes and Ketones86 Questions
Exam 15: Carboxylic Acids and Esters86 Questions
Exam 16: Amines and Amides85 Questions
Exam 17: Carbohydrates88 Questions
Exam 18: Lipids91 Questions
Exam 19: Proteins89 Questions
Exam 20: Enzymes88 Questions
Exam 21: Nucleic Acids and Protein Synthesis90 Questions
Exam 22: Nutrition and Energy for Life89 Questions
Exam 23: Carbohydrate Metabolism90 Questions
Exam 24: Lipid and Amino Acid Metabolism94 Questions
Exam 25: Body Fluids86 Questions
Select questions type
Citric acid,a natural food preservative,accounts for the tartness of citrus fruits.It is shown below.About 730 g of this material can be dissolved in water,making a liter of solution.However,only about 1.5% of it dissociates.As such,it would be considered a _____. 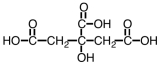
(Multiple Choice)
4.8/5  (48)
(48)
The ability to see the scattering of light when passed through a colloid is known as
(Multiple Choice)
4.8/5  (36)
(36)
A salt sample is placed into some water and nearly all of it dissolves without stirring or heating.The resulting solution is
(Multiple Choice)
4.8/5  (33)
(33)
A solution is produced in which water is the solvent and there are four solutes.Which of the solutes can dissolve better if the solution is heated?
(Multiple Choice)
4.9/5  (26)
(26)
How many moles of Na2CO3 are needed to react with 750 mL of 0.250 M H2SO4 solution? Na2CO3 + H2SO4 Na2SO4 + CO2 + H2O
(Multiple Choice)
4.8/5  (42)
(42)
In the colloid known as mayonnaise,the dispersed phase is _____ .
(Multiple Choice)
4.8/5  (42)
(42)
A solution is prepared by dissolving 4.66 g of KCl in enough distilled water to give 250 mL of solution.KCl is a strong electrolyte.How will the freezing point of the solution be different from that of pure water? (Note: Kf for water is 1.86°C/M.)
(Multiple Choice)
4.8/5  (28)
(28)
A solution is made by dissolving 5.84 grams of NaCl in enough distilled water to give a final volume of 1.00 L.What is the molarity of the solution?
(Multiple Choice)
4.9/5  (36)
(36)
The solubility of gases in water increases with increasing temperature.
(True/False)
4.9/5  (34)
(34)
When making some iced tea,you find that you can dissolve 100 grams of table sugar in a liter of tea at 20 oC.Based on this,what did you learn?
(Multiple Choice)
4.8/5  (33)
(33)
You want to remove as much CO2 gas as possible from a water solution.Which of the following treatments would be most effective?
(Multiple Choice)
4.9/5  (45)
(45)
Changes in boiling point,freezing point,and vapor pressure are
(Multiple Choice)
4.9/5  (36)
(36)
Light scattering is an effective way to distinguish between true solutions and colloidal dispersions.
(True/False)
4.9/5  (29)
(29)
When will carbon dioxide in a carbonated soft drink dissolve best?
(Multiple Choice)
4.9/5  (39)
(39)
As NH4NO3 dissolves in water,the resulting solution becomes colder.Which of the following expressions is most correct?
(Multiple Choice)
4.9/5  (42)
(42)
Which of the following pass through both osmotic and dialysis membranes?
(Multiple Choice)
4.7/5  (38)
(38)
The solubility of a substance can be measured in grams substance dissolved per liter of water.This is the same as expressing solubility in moles per liter.
(True/False)
4.8/5  (41)
(41)
There is a 12 M aqueous HCl solution in the stock room,but a 6 M solution is required for an experiment.Doubling the volume of a 12 M sample with water will produce a 6 M solution.
(True/False)
4.8/5  (36)
(36)
How many grams of solid KCl are needed to prepare 250 mL of 0.235 M solution?
(Multiple Choice)
4.8/5  (44)
(44)
When a patient's blood electrolyte levels are evaluated,sodium,chloride and bicarbonate ions are commonly measured and the difference in the total positive and negative charges calculated.This difference is called the
(Multiple Choice)
4.8/5  (36)
(36)
Showing 21 - 40 of 87
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)