Exam 8: Bonding: General Concepts
Exam 1: Chemical Foundations96 Questions
Exam 2: Atoms, molecules, and Ions91 Questions
Exam 3: Stoichiometry134 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry117 Questions
Exam 5: Gases132 Questions
Exam 6: Thermochemistry86 Questions
Exam 7: Atomic Structure and Periodicity149 Questions
Exam 8: Bonding: General Concepts146 Questions
Exam 9: Covalent Bonding: Orbitals101 Questions
Exam 10: Liquids and Solids126 Questions
Exam 11: Properties of Solutions108 Questions
Exam 12: Chemical Kinetics113 Questions
Exam 13: Chemical Equilibrium87 Questions
Exam 14: Acids and Bases149 Questions
Exam 15: Acid-Base Equilibria94 Questions
Exam 16: Solubility and Complex Ion Equilibria93 Questions
Exam 17: Spontaneity, entropy, and Free Energy117 Questions
Exam 18: Electrochemistry138 Questions
Exam 19: The Nucleus: a Chemists View83 Questions
Exam 20: The Representative Elements154 Questions
Exam 21: Transition Metals and Coordination Chemistry142 Questions
Exam 22: Organic and Biological Molecules164 Questions
Select questions type
Consider the compound crotonaldehyde,whose skeleton is: 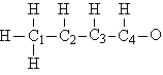 -How many nonbonding electrons appear in the Lewis structure of this molecule?
-How many nonbonding electrons appear in the Lewis structure of this molecule?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following species would be expected to have the lowest ionization energy?
(Multiple Choice)
4.8/5  (36)
(36)
Based on electronegativity differences,which of the following is most likely to be ionic?
(Multiple Choice)
4.8/5  (38)
(38)
For each of the following compounds:
a)Draw the Lewis structure.
b)Give the shape of the molecule.
c)Indicate the polarity of the molecule.
-CBrI3
(Essay)
4.9/5  (34)
(34)
As the number of bonds between two carbon atoms increases,which one of the following decreases?
(Multiple Choice)
4.8/5  (36)
(36)
How many of the following molecules-SF2,SF4,SF6,SiO2-are polar?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following molecules has a nonlinear structure?
(Multiple Choice)
4.8/5  (37)
(37)
For the elements Cs,F,and Cl,the order of increasing electronegativity is:
(Multiple Choice)
4.8/5  (28)
(28)
In the gaseous phase,which of the following diatomic molecules would be the most polar?
(Multiple Choice)
4.8/5  (42)
(42)
Which of the following atoms cannot exceed the octet rule in a molecule?
(Multiple Choice)
4.9/5  (36)
(36)
Based on electronegativities,which of the following would you expect to be most ionic?
(Multiple Choice)
4.8/5  (37)
(37)
In the cyanide ion (CN-),the nitrogen has a formal charge of
(Multiple Choice)
5.0/5  (43)
(43)
Select the correct molecular structure for the given species from the choices below:
-ClF2+
(Multiple Choice)
4.7/5  (34)
(34)
The __________ of a molecule shows how the valence electrons are arranged among the atoms in the molecule.
(Short Answer)
4.8/5  (34)
(34)
For each of the following compounds:
a)Draw the Lewis structure.
b)Give the shape of the molecule.
c)Indicate the polarity of the molecule.
-ICl4-
(Essay)
4.8/5  (38)
(38)
Showing 101 - 120 of 146
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)